|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB100030 |
|---|
|
Identification |
|---|
| Name: |
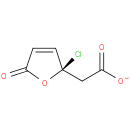
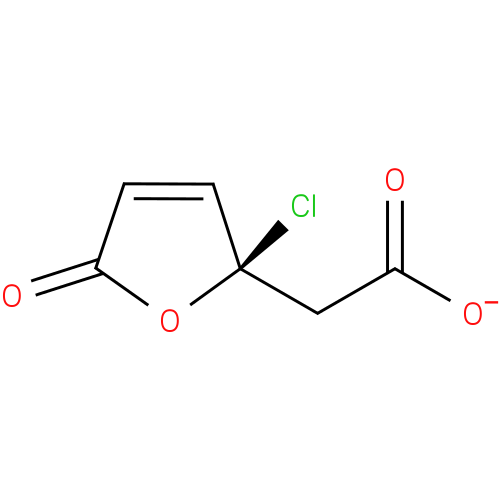
(R)-(2-chloro-5-oxo-2,5-dihydro-2-furyl)acetate |
|---|
| Description: | A (2-chloro-5-oxo-2,5-dihydro-2-furyl)acetate obtained by deprotonation of the carboxy group of (R)-(2-chloro-5-oxo-2,5-dihydro-2-furyl)acetic acid; major species at pH 7.3. |
|---|
|
Structure |
|
|---|
| Synonyms: | - (+)-4-chloromuconolactone(1−)
- (2R)-2-chloro-2,5-dihydro-5-oxofuran-2-acetate
- (R)-2-chloro-2,5-dihydro-5-oxofuran-2-acetate
|
|---|
|
Chemical Formula: |
C6H4ClO4 |
|---|
| Average Molecular Weight: |
175.547 |
|---|
| Monoisotopic Molecular
Weight: |
174.98 |
|---|
| InChI Key: |
WGZZDRVKIXVYEI-ZCFIWIBFSA-M |
|---|
| InChI: | InChI=1S/C6H5ClO4/c7-6(3-4(8)9)2-1-5(10)11-6/h1-2H,3H2,(H,8,9)/p-1/t6-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | [(2R)-2-chloro-5-oxo-2,5-dihydrofuran-2-yl]acetate |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | [O-]C(=O)C[C@]1(Cl)OC(=O)C=C1 |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as butenolides. These are dihydrofurans with a carbonyl group at the C2 carbon atom. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Dihydrofurans |
|---|
| Sub Class | Furanones |
|---|
|
Direct Parent |
Butenolides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 2-furanone
- Dicarboxylic acid or derivatives
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Carboxylic acid ester
- Lactone
- Carboxylic acid derivative
- Carboxylic acid
- Oxacycle
- Organohalogen compound
- Alkyl halide
- Hydrocarbon derivative
- Alkyl chloride
- Organic oxide
- Carbonyl group
- Organic oxygen compound
- Organochloride
- Organooxygen compound
- Organic anion
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
- (2-chloro-5-oxo-2,5-dihydro-2-furyl)acetate (CHEBI:85538)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Kajander T, Lehtiö L, Schlömann M, Goldman A. The structure of Pseudomonas P51
Cl-muconate lactonizing enzyme: co-evolution of structure and dynamics with the
dehalogenation function. Protein Sci. 2003 Sep;12(9):1855-64. Erratum in: Protein
Sci. 2003 Oct;12(10):2383. Pubmed: 12930985
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|