|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB100029 |
|---|
|
Identification |
|---|
| Name: |
pyrrolnitrin |
|---|
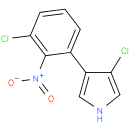
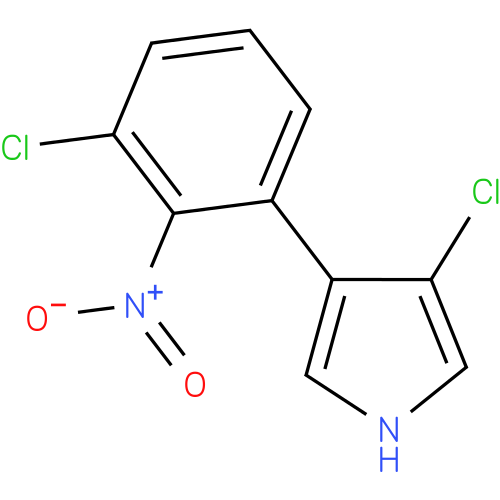
| Description: | A member of the class of pyrroles carrying chloro and 3-chloro-2-nitrophenyl substituents at positions 3 and 4 respectively. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 3-Chloro-4-(2'-nitro-3'-chlorophenyl)pyrrole
- 3-Chloro-4-(3-chloro-2-nitrophenyl)pyrrole
- NSC 107654
- NSC-107654
- pyrrolnitrin
|
|---|
|
Chemical Formula: |
C10H6Cl2N2O2 |
|---|
| Average Molecular Weight: |
257.073 |
|---|
| Monoisotopic Molecular
Weight: |
255.981 |
|---|
| InChI Key: |
QJBZDBLBQWFTPZ-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C10H6Cl2N2O2/c11-8-3-1-2-6(10(8)14(15)16)7-4-13-5-9(7)12/h1-5,13H |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 3-chloro-4-(3-chloro-2-nitrophenyl)-1H-pyrrole |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | [O-][N+](=O)c1c(Cl)cccc1-c1c[nH]cc1Cl |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as phenylpyrroles. These are polycyclic aromatic compounds containing a benzene ring linked to a pyrrole ring through a CC or CN bond. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Pyrroles |
|---|
| Sub Class | Substituted pyrroles |
|---|
|
Direct Parent |
Phenylpyrroles |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 3-phenylpyrrole
- Nitrobenzene
- Nitroaromatic compound
- Chlorobenzene
- Halobenzene
- Aryl chloride
- Aryl halide
- Monocyclic benzene moiety
- Benzenoid
- Heteroaromatic compound
- Organic nitro compound
- C-nitro compound
- Propargyl-type 1,3-dipolar organic compound
- Allyl-type 1,3-dipolar organic compound
- Azacycle
- Organic oxoazanium
- Organic 1,3-dipolar compound
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
- C-nitro compound, indole alkaloid, monochlorobenzenes, ring assembly, pyrroles (CHEBI:32079)
- a chloroaromatic compound (CPD-12776)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Thongsri Y, Aromdee C, Yenjai C, Kanokmedhakul S, Chaiprasert A, Hamal P,
Prariyachatigul C. Detection of diketopiperazine and pyrrolnitrin, compounds with
anti-Pythium insidiosum activity, in a Pseudomonas stutzeri environmental strain.
Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014 Sep;158(3):378-83. Pubmed: 23149469
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|