|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB100017 |
|---|
|
Identification |
|---|
| Name: |
monodechloroaminopyrrolnitrin |
|---|
| Description: | A member of the class of pyrroles carrying a 2-amino-3-chlorophenyl substituent at position 3. |
|---|
|
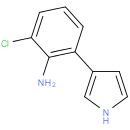
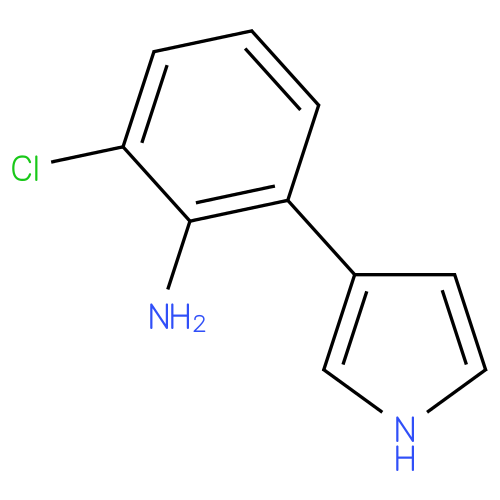
Structure |
|
|---|
| Synonyms: | - 2-chloro-6-(1H-pyrrol-3-yl)benzenamine
- 2-chloro-6-(pyrrol-3-yl)aniline
- 4-(2-amino-3-chlorophenyl)pyrrole
- monodechloroaminopyrrolnitrin
|
|---|
|
Chemical Formula: |
C10H9ClN2 |
|---|
| Average Molecular Weight: |
192.645 |
|---|
| Monoisotopic Molecular
Weight: |
192.045 |
|---|
| InChI Key: |
VLWKKIHFPGKVHZ-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C10H9ClN2/c11-9-3-1-2-8(10(9)12)7-4-5-13-6-7/h1-6,13H,12H2 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 2-chloro-6-(1H-pyrrol-3-yl)aniline |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | Nc1c(Cl)cccc1-c1cc[nH]c1 |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as phenylpyrroles. These are polycyclic aromatic compounds containing a benzene ring linked to a pyrrole ring through a CC or CN bond. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Pyrroles |
|---|
| Sub Class | Substituted pyrroles |
|---|
|
Direct Parent |
Phenylpyrroles |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 3-phenylpyrrole
- Aniline or substituted anilines
- Chlorobenzene
- Halobenzene
- Aryl chloride
- Aryl halide
- Monocyclic benzene moiety
- Benzenoid
- Heteroaromatic compound
- Azacycle
- Hydrocarbon derivative
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Organopnictogen compound
- Organic nitrogen compound
- Amine
- Primary amine
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
- substituted aniline, indole alkaloid, monochlorobenzenes, ring assembly, pyrroles (CHEBI:85785)
- a chloroaromatic compound (CPD-12774)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- De Laurentis W, Khim L, Anderson JL, Adam A, Johnson KA, Phillips RS, Chapman
SK, van Pee KH, Naismith JH. The second enzyme in pyrrolnitrin biosynthetic
pathway is related to the heme-dependent dioxygenase superfamily. Biochemistry.
2007 Oct 30;46(43):12393-404. Pubmed: 17924666
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|