|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB100012 |
|---|
|
Identification |
|---|
| Name: |
iminoarginine |
|---|
| Description: | A dehydroamino acid that is arginine in which the amino group has been oxidised to the corresponding imine. |
|---|
|
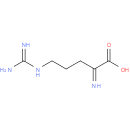
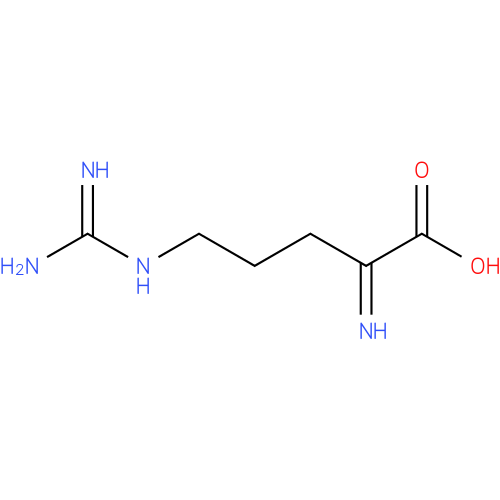
Structure |
|
|---|
| Synonyms: | Not Available |
|---|
|
Chemical Formula: |
C6H12N4O2 |
|---|
| Average Molecular Weight: |
172.1851 |
|---|
| Monoisotopic Molecular
Weight: |
172.096 |
|---|
| InChI Key: |
YWGYOCPWFDUKSA-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C6H12N4O2/c7-4(5(11)12)2-1-3-10-6(8)9/h7H,1-3H2,(H,11,12)(H4,8,9,10) |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 5-carbamimidamido-2-iminopentanoic acid |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | NC(=N)NCCCC(=N)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as fatty acids and conjugates. These are aliphatic monocarboxylic acids with a saturated or unsaturated aliphatic tail (with at least 4 Carbon atoms). |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
|
Direct Parent |
Fatty acids and conjugates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Fatty acid
- Guanidine
- Ketimine
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboximidamide
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxide
- Hydrocarbon derivative
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Imine
- Organopnictogen compound
- Organic oxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
- guanidines, dehydroamino acid, ketimine, arginine derivative (CHEBI:84259)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Gannavaram S, Sirin S, Sherman W, Gadda G. Mechanistic and computational
studies of the reductive half-reaction of tyrosine to phenylalanine active site
variants of D-arginine dehydrogenase. Biochemistry. 2014 Oct 21;53(41):6574-83.
Pubmed: 25243743
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|