|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB100009 |
|---|
|
Identification |
|---|
| Name: |
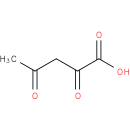
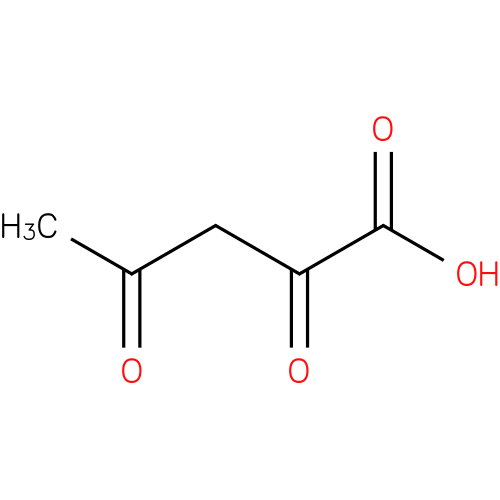
acetylpyruvic acid |
|---|
| Description: | A dioxo monocarboxylic acid that is pentanoic acid carrying two oxo groups at positions 2 and 4. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 2,4-Dioxopentanoate
- 2,4-dioxovaleric acid
- Acetylpyruvate
- Acetylpyruvic acid
|
|---|
|
Chemical Formula: |
C5H6O4 |
|---|
| Average Molecular Weight: |
130.09874 |
|---|
| Monoisotopic Molecular
Weight: |
130.027 |
|---|
| InChI Key: |
UNRQTHVKJQUDDF-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C5H6O4/c1-3(6)2-4(7)5(8)9/h2H2,1H3,(H,8,9) |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 2,4-dioxopentanoic acid |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CC(=O)CC(=O)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as 3-acylpyruvic acids. These are compounds containing a pyruvic acid substituted at the 3-position by an acyl group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Keto acids and derivatives |
|---|
| Sub Class | Gamma-keto acids and derivatives |
|---|
|
Direct Parent |
3-Acylpyruvic acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 3-acylpyruvic acid
- 1,3-diketone
- Short-chain keto acid
- Alpha-keto acid
- 1,3-dicarbonyl compound
- Alpha-hydroxy ketone
- Ketone
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
- dioxo monocarboxylic acid, beta-diketone (CHEBI:2424)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Chapman PJ, Ribbons DW. Metabolism of resorcinylic compounds by bacteria:
orcinol pathway in Pseudomonas putida. J Bacteriol. 1976 Mar;125(3):975-84. Pubmed: 1254564
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| KEGG COMPOUND | C02132 |
|
|---|