|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB100002 |
|---|
|
Identification |
|---|
| Name: |
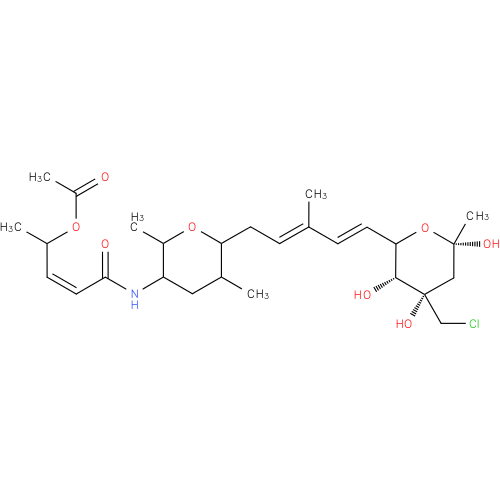
FR901463 |
|---|
| Description: | A cyclic hemiketal isolated from the fermentation broth of Pseudomonas sp.no.2663. It displays potent cytotoxic activity against human tumour cells. |
|---|
|
Structure |
|
|---|
| Synonyms: | - (3Z)-5-[(6-{(2E,4E)-5-[(3R,4S,6S)-4-(chloromethyl)-3,4,6-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl]-3-methylpenta-2,4-dien-1-yl}-2,5-dimethyltetrahydro-2H-pyran-3-yl)amino]-5-oxopent-3-en-2-yl acetate
|
|---|
|
Chemical Formula: |
C27H42ClNO8 |
|---|
| Average Molecular Weight: |
544.077 |
|---|
| Monoisotopic Molecular
Weight: |
543.26 |
|---|
| InChI Key: |
RONUKPQOBQKEHX-FOUIGEGESA-N |
|---|
| InChI: | - InChI=1S/C27H42ClNO8/c1-16(8-11-23-25(32)27(34,15-28)14-26(6,33)37-23)7-10-22-17(2)13-21(19(4)36-22)29-24(31)12-9-18(3)35-20(5)30/h7-9,11-12,17-19,21-23,25,32-34H,10,13-15H2,1-6H3,(H,29,31)/b11-8+,12-9-,16-7+/t17?,18?,19?,21?,22?,23?,25-,26+,27-/m1/s1
|
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 6-[(1E,3E)-5-(5-{[(2Z)-4-(acetyloxy)pent-2-enoyl]amino}-3,6-dimethyltetrahydro-2H-pyran-2-yl)-3-methylpenta-1,3-dien-1-yl]-4-C-(chloromethyl)-1,3-dideoxy-a-D-erythro-hex-2-ulopyranose |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CC1CC(NC(=O)\C=C/C(C)OC(C)=O)C(C)OC1C\C=C(C)\C=C\C1O[C@](C)(O)C[C@@](O)(CCl)[C@@H]1O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as n-acyl amines. These are compounds containing a fatty acid moiety linked to an amine group through an ester linkage. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Fatty Acyls |
|---|
| Sub Class | Fatty amides |
|---|
|
Direct Parent |
N-acyl amines |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Monosaccharide
- N-acyl-amine
- Oxane
- Tertiary alcohol
- 1,2-diol
- Carboxamide group
- Carboxylic acid ester
- Chlorohydrin
- Halohydrin
- Hemiacetal
- Secondary alcohol
- Secondary carboxylic acid amide
- Oxacycle
- Organoheterocyclic compound
- Carboxylic acid derivative
- Polyol
- Dialkyl ether
- Ether
- Monocarboxylic acid or derivatives
- Hydrocarbon derivative
- Carbonyl group
- Alkyl halide
- Organic nitrogen compound
- Organic oxygen compound
- Alkyl chloride
- Organopnictogen compound
- Organohalogen compound
- Alcohol
- Organochloride
- Organic oxide
- Organonitrogen compound
- Organooxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
- monocarboxylic acid amide, organochlorine compound, acetate ester, cyclic hemiketal, pyrans (CHEBI:65914)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Nakajima, Hidenori, et al. "New antitumor substances, FR901463, FR901464 and FR901465. I. Taxonomy, fermentation, isolation, physico-chemical properties and biological activities." The Journal of antibiotics 49.12 (1996): 1196-1203. Pubmed: 9031664
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
Not Available |
|---|