|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB100001 |
|---|
|
Identification |
|---|
| Name: |
spiruchostatin B |
|---|
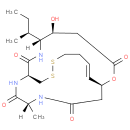
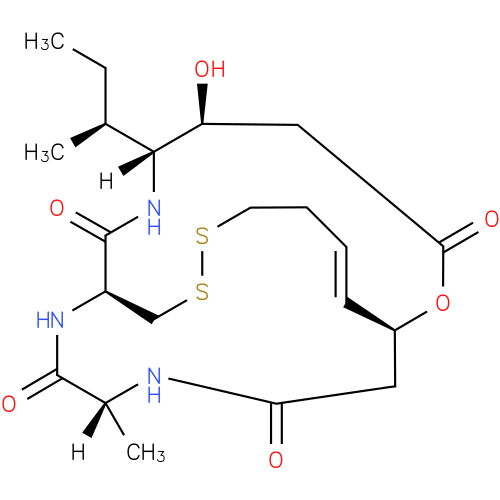
| Description: | A spiruchostatin with molecular formula C21H33N3O6S2 originally isolated from a Pseudomonas culture broth. |
|---|
|
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C21H33N3O6S2 |
|---|
| Average Molecular Weight: |
487.636 |
|---|
| Monoisotopic Molecular
Weight: |
487.181 |
|---|
| InChI Key: |
MJHZJODQLYCXHE-WXZCCWHXSA-N |
|---|
| InChI: | InChI=1S/C20H31N3O6S2/c1-11(2)18-15(24)9-17(26)29-13-6-4-5-7-30-31-10-14(20(28)23-18)22-19(27)12(3)21-16(25)8-13/h4,6,11-15,18,24H,5,7-10H2,1-3H3,(H,21,25)(H,22,27)(H,23,28)/b6-4-/t12-,13-,14-,15+,18-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (1S,5S,6R,9S,15Z,20R)-5-hydroxy-20-methyl-6-propan-2-yl-2-oxa-11,12-dithia-7,19,22-triazabicyclo[7.7.6]docos-15-ene-3,8,18,21-tetrone |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | C1SSCCC=C[C@@H]2CC(N[C@@](C(N[C@H]1C(N[C@@]([C@H](CC(O2)=O)O)([C@H](CC)C)[H])=O)=O)(C)[H])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as cyclic depsipeptides. These are natural or synthetic compounds having sequences of amino and hydroxy carboxylic acid residues (usually ??-amino and ??-hydroxy acids) connected in a ring. The residues are commonly but not necessarily regularly alternating. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Peptidomimetics |
|---|
| Sub Class | Depsipeptides |
|---|
|
Direct Parent |
Cyclic depsipeptides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha-dipeptide
- Cyclic depsipeptide
- Macrolide lactam
- Macrolactam
- Macrolide
- Alpha-amino acid or derivatives
- Carboxamide group
- Carboxylic acid ester
- Lactam
- Lactone
- Organic disulfide
- Secondary alcohol
- Secondary carboxylic acid amide
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Carbonyl group
- Organic nitrogen compound
- Organic oxygen compound
- Organonitrogen compound
- Organooxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Takizawa, Toshiya, et al. "Total synthesis of spiruchostatin B, a potent histone deacetylase inhibitor, from a microorganism." Chemical Communications 14 (2008): 1677-1679. Pubmed: 18368162
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
Not Available |
|---|