|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB100000 |
|---|
|
Identification |
|---|
| Name: |
karalicin |
|---|
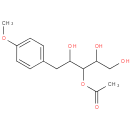
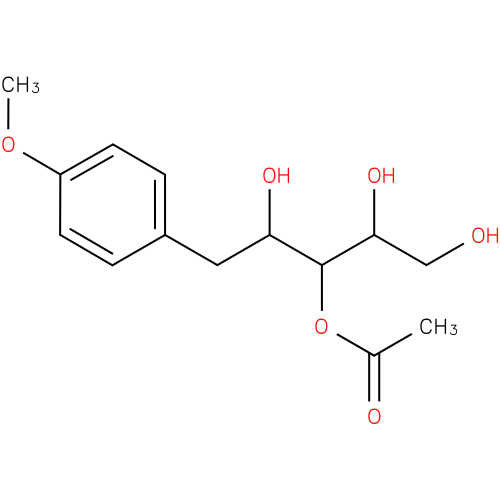
| Description: | A pentitol derivative that is 3-O-acetyl-1-deoxypentitol substituted by a 4-methoxyphenyl group at position 1. Isolated from the fermentation broth of Pseudomonas fluorescens and Pseudomonas putida, it exhibits anti-HSV-1 activity. |
|---|
|
Structure |
|
|---|
| Synonyms: | - [1,2,4-trihydroxy-5-(4-methoxyphenyl)pentan-3-yl] acetate
|
|---|
|
Chemical Formula: |
C14H20O6 |
|---|
| Average Molecular Weight: |
284.305 |
|---|
| Monoisotopic Molecular
Weight: |
284.126 |
|---|
| InChI Key: |
JATBUOZGJQJSGA-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C14H20O6/c1-9(16)20-14(13(18)8-15)12(17)7-10-3-5-11(19-2)6-4-10/h3-6,12-15,17-18H,7-8H2,1-2H3 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 3-O-acetyl-1-deoxy-1-(4-methoxyphenyl)pentitol |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | COc1ccc(CC(O)C(OC(C)=O)C(O)CO)cc1 |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as fatty alcohols. These are aliphatic alcohols consisting of a chain of a least six carbon atoms. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Fatty Acyls |
|---|
| Sub Class | Fatty alcohols |
|---|
|
Direct Parent |
Fatty alcohols |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Fatty alcohol
- Phenoxy compound
- Anisole
- Methoxybenzene
- Phenol ether
- Alkyl aryl ether
- Monocyclic benzene moiety
- Benzenoid
- Secondary alcohol
- Carboxylic acid ester
- Carboxylic acid derivative
- Polyol
- Monocarboxylic acid or derivatives
- Ether
- Alcohol
- Organooxygen compound
- Primary alcohol
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
- acetate ester, monomethoxybenzene, triol, pentitol derivative (CHEBI:66140)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Lampis, G., et al. "Karalicin, a new biologically active compound from Pseudomonas fluorescens/putida." The Journal of antibiotics 49.3 (1996): 263-266. Pubmed: 8626242
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
Not Available |
|---|