|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB006991 |
|---|
|
Identification |
|---|
| Name: |
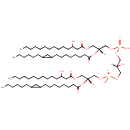
CL(14:0(3-OH)/17:0cycw7c/14:0(3-OH)/17:0cycw7c) |
|---|
| Description: | CL(14:0(3-OH)/17:0cycw7c/14:0(3-OH)/17:0cycw7c) is a cardiolipin (CL). Cardiolipins are sometimes called a 'double' phospholipid because they have four fatty acid tails, instead of the usual two. CL(14:0(3-OH)/17:0cycw7c/14:0(3-OH)/17:0cycw7c) contains two chains of 3-hydroxytetradecanoic acid at the C1 and C3 positions, two chains of (heptadec-9-10-cyclo-anoyl) at the C2 and C4 positions. While the theoretical charge of cardiolipins is -2, under normal physiological conditions (pH near 7), the molecule may carry only one negative charge. In prokaryotes such as Pseudomonas aeruginosa, the enzyme known as diphosphatidylglycerol synthase catalyses the transfer of the phosphatidyl moiety of one phosphatidylglycerol to the free 3'-hydroxyl group of another, with the elimination of one molecule of glycerol. In Pseudomonas aeruginosa, which acylates its glycerophospholipids with acyl chains ranging in length from 12 to 19 carbons and possibly containing an unsaturation, or a cyclopropane group more than 100 possible CL molecular species are theoretically possible. Pseudomonas aeruginosa membranes consist of ~5% cardiolipin (CL), 20-25% phosphatidylglycerol (PG), and 70-80% phosphatidylethanolamine (PE) as well as smaller amounts of phosphatidylserine (PS). CL is distributed between the two leaflets of the bilayers and is located preferentially at the poles and septa in Pseudomonas aeruginosa and other rod-shaped bacteria. It is known that the polar positioning of the proline transporter ProP and the mechanosensitive ion channel MscS in Pseudomonas aeruginosa is dependent on CL. It is believed that cell shape may influence the localization of CL and the localization of certain membrane proteins. |
|---|
|
Structure |
|
|---|
| Synonyms: | Not Available |
|---|
|
Chemical Formula: |
C71H134O19P2 |
|---|
| Average Molecular Weight: |
1353.782 |
|---|
| Monoisotopic Molecular
Weight: |
1352.899456091 |
|---|
| InChI Key: |
PXBFQDSMQNXKDZ-GCHYIDFVSA-N |
|---|
| InChI: | InChI=1S/C71H134O19P2/c1-5-9-13-17-19-21-23-29-37-45-63(72)51-70(77)83-55-66(89-68(75)47-39-31-25-27-35-43-61-49-59(61)41-33-15-11-7-3)57-87-91(79,80)85-53-65(74)54-86-92(81,82)88-58-67(56-84-71(78)52-64(73)46-38-30-24-22-20-18-14-10-6-2)90-69(76)48-40-32-26-28-36-44-62-50-60(62)42-34-16-12-8-4/h59-67,72-74H,5-58H2,1-4H3,(H,79,80)(H,81,82)/t59?,60?,61?,62?,63?,64?,65?,66-,67-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | [(2R)-2-{[8-(2-hexylcyclopropyl)octanoyl]oxy}-3-[(3-hydroxytetradecanoyl)oxy]propoxy][3-({[(2R)-2-{[8-(2-hexylcyclopropyl)octanoyl]oxy}-3-[(3-hydroxytetradecanoyl)oxy]propoxy](hydroxy)phosphoryl}oxy)-2-hydroxypropoxy]phosphinic acid |
|---|
|
Traditional IUPAC Name: |
(2R)-2-{[8-(2-hexylcyclopropyl)octanoyl]oxy}-3-[(3-hydroxytetradecanoyl)oxy]propoxy(3-{[(2R)-2-{[8-(2-hexylcyclopropyl)octanoyl]oxy}-3-[(3-hydroxytetradecanoyl)oxy]propoxy(hydroxy)phosphoryl]oxy}-2-hydroxypropoxy)phosphinic acid |
|---|
| SMILES: | CCCCCCCCCCCC(O)CC(=O)OC[C@H](COP(O)(=O)OCC(O)COP(O)(=O)OC[C@@H](COC(=O)CC(O)CCCCCCCCCCC)OC(=O)CCCCCCCC1CC1CCCCCC)OC(=O)CCCCCCCC1CC1CCCCCC |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as cardiolipins. These are glycerophospholipids in which the O1 and O3 oxygen atoms of the central glycerol moiety are each linked to one 1,2-diacylglycerol chain. Their general formula is OC(COP(O)(=O)OC[C@@H](CO[R1])O[R2])COP(O)(=O)OC[C@@H](CO[R3])O[R4], where R1-R4 are four fatty acyl chains. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Glycerophospholipids |
|---|
| Sub Class | Glycerophosphoglycerophosphoglycerols |
|---|
|
Direct Parent |
Cardiolipins |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Cardiolipin
- Tetracarboxylic acid or derivatives
- Beta-hydroxy acid
- Fatty acid ester
- Dialkyl phosphate
- Fatty acyl
- Hydroxy acid
- Organic phosphate
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Secondary alcohol
- Carboxylic acid ester
- Carboxylic acid derivative
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organooxygen compound
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic homomonocyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Membrane |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- De Siervo, A. J. (1969). "Alterations in the phospholipid composition of Escherichia coli B during growth at different temperatures." J Bacteriol 100:1342-1349. Pubmed: 4902814
- Garrett, T. A., O'Neill, A. C., Hopson, M. L. (2012). "Quantification of cardiolipin molecular species in Escherichia coli lipid extracts using liquid chromatography/electrospray ionization mass spectrometry." Rapid Commun Mass Spectrom 26:2267-2274. Pubmed: 22956318
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Uniprot Consortium (2012). "Reorganizing the protein space at the Universal Protein Resource (UniProt)." Nucleic Acids Res 40:D71-D75. Pubmed: 22102590
- Yurtsever D. (2007). Fatty acid methyl ester profiling of Enterococcus and Esherichia coli for microbial source tracking. M.sc. Thesis. Villanova University: U.S.A
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | Not Available | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|