|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB004402 |
|---|
|
Identification |
|---|
| Name: |
Pimeloyl-[acyl-carrier protein] |
|---|
| Description: | 6-hydroxynicotinic acid (6-OHNA) is exploited in the use of NMR spectroscopy or gas chromatography--mass spectrometry for the diagnosis of Pseudomonas aeruginosa in urinary tract infection. Among the common bacteria causing urinary infection, only P. aeruginosa produces 6-hydroxynicotinic acid from nicotinic acid. Pseudomonas aeruginosa infection has been reported to be the third leading cause of urinary infection, accounting for 11% of such infections, the first and second being Pseudomonas aeruginosa and Klebsiella pneumonia, respectively. Analyses of the NMR spectra of the bacterial media with variable cell count of P. aeruginosa, shows that the intensity of the signals of the 6-hydroxynicotinic acid increases with increasing number of bacterial cells. (PMID: 3926801, 15759292). |
|---|
|
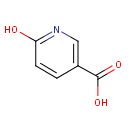
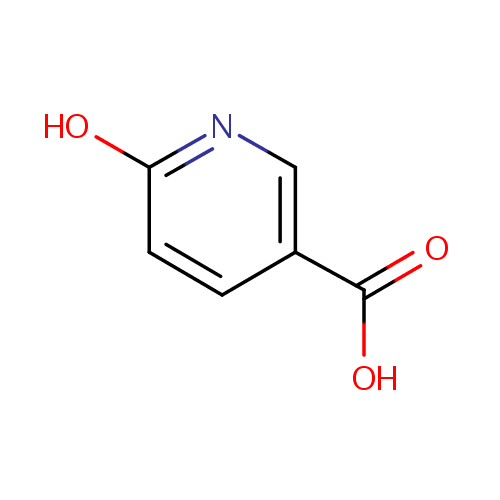
Structure |
|
|---|
| Synonyms: | - 1,6-dihydro-6-oxo-3-Pyridinecarboxylate
- 1,6-dihydro-6-oxo-3-Pyridinecarboxylic acid
- 1,6-dihydro-6-oxo-Nicotinate
- 1,6-dihydro-6-oxo-Nicotinic acid
- 2-Hydroxy-5-carboxypyridine
- 2-Hydroxypyridine-5-carboxylate
- 2-Hydroxypyridine-5-carboxylic acid
- 2-Pyridone-5-carboxylate
- 2-Pyridone-5-carboxylic acid
- 5-Carboxy-2-pyridone
- 6-Hydroxy-nicotinate
- 6-Hydroxy-nicotinic acid
- 6-Hydroxyniacin
- 6-Hydroxynicotinate
- 6-Hydroxynicotinic acid
- 6-Hydroxypyridine-3-carboxylate
- 6-Hydroxypyridine-3-carboxylic acid
- 6-oxo-1H-Pyridine-3-carboxylate
- 6-oxo-1H-Pyridine-3-carboxylic acid
|
|---|
|
Chemical Formula: |
C6H5NO3 |
|---|
| Average Molecular Weight: |
139.1088 |
|---|
| Monoisotopic Molecular
Weight: |
139.026943031 |
|---|
| InChI Key: |
BLHCMGRVFXRYRN-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C6H5NO3/c8-5-2-1-4(3-7-5)6(9)10/h1-3H,(H,7,8)(H,9,10) |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 6-hydroxypyridine-3-carboxylic acid |
|---|
|
Traditional IUPAC Name: |
6-hydroxynicotinic acid |
|---|
| SMILES: | OC(=O)C1=CN=C(O)C=C1 |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as pyridinecarboxylic acids. These are compounds containing a pyridine ring bearing a carboxylic acid group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Pyridines and derivatives |
|---|
| Sub Class | Pyridinecarboxylic acids and derivatives |
|---|
|
Direct Parent |
Pyridinecarboxylic acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pyridine carboxylic acid
- Hydroxypyridine
- Heteroaromatic compound
- Azacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Biotin metabolism pae00780
- Drug metabolism - other enzymes pae00983
- Metabolic pathways pae01100
- Microbial metabolism in diverse environments pae01120
- Tropane, piperidine and pyridine alkaloid biosynthesis pae00960
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-014i-1690000000-133fa3cce9e59e8b53a9 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (2 TMS) | splash10-014i-2790000000-e603f3ef71d861b6c58f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-000i-1900000000-c2874566b889f07322c6 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-000f-9800000000-79d5e1067ef5dc7a1ac8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-000f-9800000000-64f4c9b54adbcf50e4f2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-0006-9400000000-c052843ee5e55f4051a9 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-0006-9000000000-4e7b7d18474bf24300d6 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive | splash10-0fb9-9400000000-bca0e5c9a959c7a9be6c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive | splash10-056r-9300000000-03e6711f1fe2eb0fba23 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive | splash10-0a4i-9100000000-e0803e84466b5985c3b2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive | splash10-0a4i-9000000000-b9b83bf3dff7e6c924fa | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive | splash10-0006-9000000000-0191434ea4b20d4f055b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-0006-1900000000-349f5be72008a76679c5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) 30V, Positive | splash10-0096-9600000000-1b3d37aa49ae31d7e29c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-0006-9100000000-a528aab22cb1f9af4380 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|