|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB004396 |
|---|
|
Identification |
|---|
| Name: |
L-Prolyl-tRNA(Pro) |
|---|
| Description: | N-Carbamoylsarcosine is an intermediate in arginine and proline metabolism. It is also involved in a metabolic pathway for the degradation of creatinine. In this pathway, creatinine is not hydrolyzed back to creatine. Instead, it is deaminated to N-methylhydantoin, releasing an amonia molecule, by the action of creatinine deaminase (also known as creatinine iminohydrolase). N-methylhydantoin is then hydrolyzed to N-carbamoylsarcosine, by the action of N-methylhydantoin amidohydrolase, at the expense of one ATP molecule. N-carbamoylsarcosine is deaminated further to sarcosine by N-carbamoylsarcosine amidohydrolase, releasing a second ammonia molecule. In the last step of this pathway, sarcosine is hydrolyzed to glycine and formaldehyde, by either sarcosine dehydrogenase or sarcosine oxidase. |
|---|
|
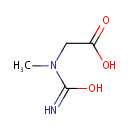
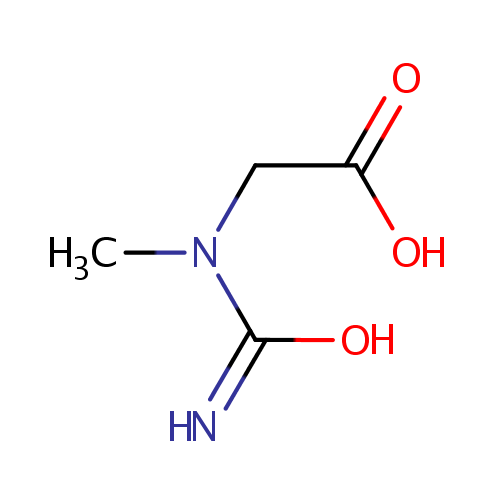
Structure |
|
|---|
| Synonyms: | - Carbamoyl sarcosine
- Carbamoyl-sarcosine
- CMS
- [Carbamoyl(methyl)amino]acetate
- [Carbamoyl(methyl)amino]acetic acid
|
|---|
|
Chemical Formula: |
C4H8N2O3 |
|---|
| Average Molecular Weight: |
132.1179 |
|---|
| Monoisotopic Molecular
Weight: |
132.053492132 |
|---|
| InChI Key: |
SREKYKXYSQMOIB-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C4H8N2O3/c1-6(4(5)9)2-3(7)8/h2H2,1H3,(H2,5,9)(H,7,8) |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 2-[(C-hydroxycarbonimidoyl)(methyl)amino]acetic acid |
|---|
|
Traditional IUPAC Name: |
[C-hydroxycarbonimidoyl(methyl)amino]acetic acid |
|---|
| SMILES: | CN(CC(O)=O)C(O)=N |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as alpha amino acids and derivatives. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon), or a derivative thereof. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
|
Direct Parent |
Alpha amino acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha-amino acid or derivatives
- Urea
- Tertiary amine
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|