|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB004273 |
|---|
|
Identification |
|---|
| Name: |
L-Histidyl-tRNA(His) |
|---|
| Description: | L-Histidyl-tRNA(His) is an intermediate in aminoacyl-tRNA biosynthesis in E.coli. It is a product for the enzyme histidyl tRNA synthetase which catalyzes the reaction a tRNAhis + L-histidine -> an L-histidyl-[tRNAhis] (KEGG compound: C02988). |
|---|
|
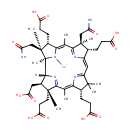
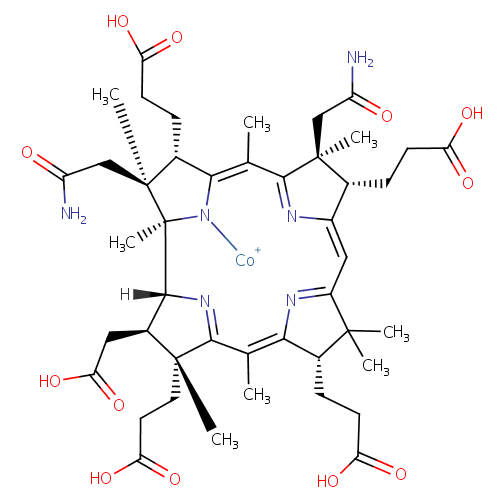
Structure |
|
|---|
| Synonyms: | - Cob(II)yrinate a,c diamide
- Cob(II)yrinate a,c-diamide
- Cob(II)yrinate diamide
- Cob(ii)yrinic acid a,c diamide
- Cob(II)yrinic acid a,c-diamide
- Cob(II)yrinic acid diamide
|
|---|
|
Chemical Formula: |
C45H61CoN6O12 |
|---|
| Average Molecular Weight: |
936.946 |
|---|
| Monoisotopic Molecular
Weight: |
936.367392 |
|---|
| InChI Key: |
IADMSJRJSGLGJI-UHFFFAOYSA-M |
|---|
| InChI: | InChI=1S/C45H62N6O12.Co/c1-21-36-24(10-13-32(56)57)41(3,4)28(49-36)18-27-23(9-12-31(54)55)43(6,19-29(46)52)39(48-27)22(2)37-25(11-14-33(58)59)44(7,20-30(47)53)45(8,51-37)40-26(17-35(62)63)42(5,38(21)50-40)16-15-34(60)61;/h18,23-26,40H,9-17,19-20H2,1-8H3,(H10,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63);/q;+2/p-1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | [(1R,2R,3R,4R,6Z,8S,13S,14S,18S,19S)-14,19-bis(carbamoylmethyl)-4,8,13,18-tetrakis(2-carboxyethyl)-3-(carboxymethyl)-1,4,6,9,9,14,16,19-octamethyl-20,21,22,23-tetraazapentacyclo[15.2.1.1?,??1?????1??,???tricosa-5(23),6,10(22),11,15(21),16-hexaen-20-yl]cobaltylium |
|---|
|
Traditional IUPAC Name: |
[(1R,2R,3R,4R,6Z,8S,13S,14S,18S,19S)-14,19-bis(carbamoylmethyl)-4,8,13,18-tetrakis(2-carboxyethyl)-3-(carboxymethyl)-1,4,6,9,9,14,16,19-octamethyl-20,21,22,23-tetraazapentacyclo[15.2.1.1?,??1?????1??,???tricosa-5(23),6,10(22),11,15(21),16-hexaen-20-yl]cobaltylium |
|---|
| SMILES: | [Co++].[H]C1(CCC(O)=O)\C2=C\C3=N\C(=C(C)\C4=NC([H])(C([H])(CC(O)=O)C4(C)CCC(O)=O)C4(C)N\C(=C(C)/C(=N2)C1(C)CC([NH-])=O)C([H])(CCC(O)=O)C4(C)CC(O)=N)\C([H])(CCC(O)=O)C3(C)C |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | Not Available |
|---|
|
Kingdom |
Not Available |
|---|
| Super Class | Not Available |
|---|
|
Class |
Not Available |
|---|
| Sub Class | Not Available |
|---|
|
Direct Parent |
Not Available |
|---|
| Alternative Parents |
Not Available |
|---|
| Substituents |
Not Available |
|---|
| Molecular Framework |
Not Available |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | 27937 | | HMDB ID | Not Available | | Pubchem Compound ID | 11953882 | | Kegg ID | C02988 | | ChemSpider ID | 10128181 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|