|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB004183 |
|---|
|
Identification |
|---|
| Name: |
4-Hydroxy-3-polyprenylbenzoate |
|---|
| Description: | A member of the class of benzoates obtained by deprotonation of the carboxy group of any 4-hydroxy-3-polyprenylbenzoic acid; major species at pH 7.3 |
|---|
|
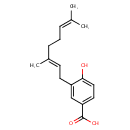
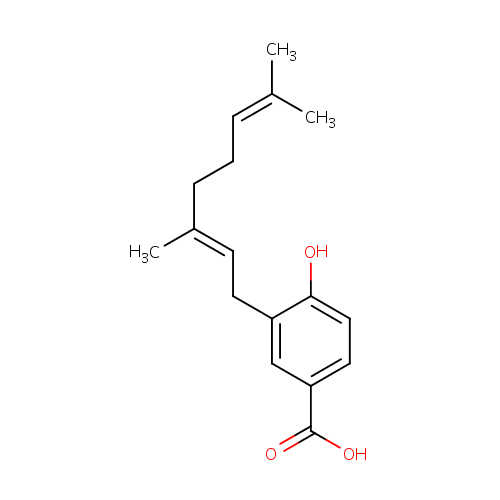
Structure |
|
|---|
| Synonyms: | - 4-Hydroxy-3-polyprenylbenzoic acid
|
|---|
|
Chemical Formula: |
C17H22O3 |
|---|
| Average Molecular Weight: |
274.3548 |
|---|
| Monoisotopic Molecular
Weight: |
274.15689457 |
|---|
| InChI Key: |
HKIMBCGCVPYUTJ-NTUHNPAUSA-N |
|---|
| InChI: | InChI=1S/C17H22O3/c1-12(2)5-4-6-13(3)7-8-14-11-15(17(19)20)9-10-16(14)18/h5,7,9-11,18H,4,6,8H2,1-3H3,(H,19,20)/b13-7+ |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 3-[(2E)-3,7-dimethylocta-2,6-dien-1-yl]-4-hydroxybenzoic acid |
|---|
|
Traditional IUPAC Name: |
4-hydroxy-3-polyprenylbenzoate |
|---|
| SMILES: | CC(C)=CCC\C(C)=C\CC1=CC(=CC=C1O)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as aromatic monoterpenoids. These are monoterpenoids containing at least one aromatic ring. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Prenol lipids |
|---|
| Sub Class | Monoterpenoids |
|---|
|
Direct Parent |
Aromatic monoterpenoids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Monocyclic monoterpenoid
- Hydroxybenzoic acid
- Aromatic monoterpenoid
- Benzoic acid
- Benzoic acid or derivatives
- Benzoyl
- Phenol
- Benzenoid
- Monocyclic benzene moiety
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Membrane |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Metabolic pathways pae01100
- Ubiquinone and other terpenoid-quinone biosynthesis pae00130
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|