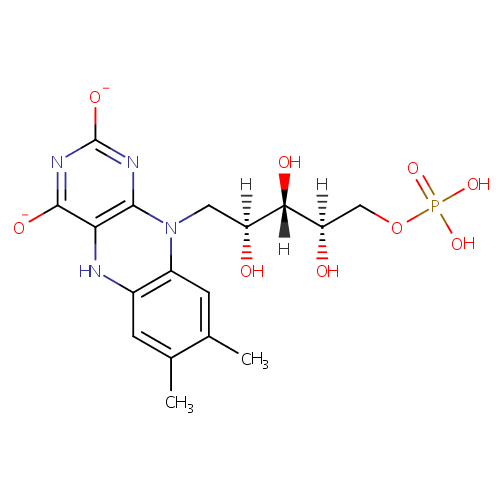
FMNH(2) (PAMDB003577)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB003577 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | FMNH(2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | FMNH2 is the reduced form of flavin mononucleotide. It is a substrate of the enzyme FMN reductase (EC 1.5.1.29), an enzyme that catalyzes the chemical reaction FMNH2 + NAD(P)+ <=> FMN + NAD(P)H + H+. Flavin mononucleotide (FMN), or riboflavin-5??phosphate, is a biomolecule produced from riboflavin (vitamin B2) by the enzyme riboflavin kinase and functions as prosthetic group of various oxidoreductases including NADH dehydrogenase. During a catalytic cycle, the reversible interconversion of oxidized (FMN), semiquinone (FMNH?? and reduced (FMNH2) forms occurs in the various oxidoreductases. FMN is a stronger oxidizing agent than NAD and is particularly useful because it can take part in both one- and two-electron transfers. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C17H21N4O9P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 456.3438 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 456.104614802 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | YTNIXZGTHTVJBW-SCRDCRAPSA-L | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C17H23N4O9P/c1-7-3-9-10(4-8(7)2)21(15-13(18-9)16(25)20-17(26)19-15)5-11(22)14(24)12(23)6-30-31(27,28)29/h3-4,11-12,14,18,22-24H,5-6H2,1-2H3,(H2,27,28,29)(H2,19,20,25,26)/p-2/t11-,12+,14-/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 5666-16-0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 7,8-dimethyl-10-[(2S,3S,4R)-2,3,4-trihydroxy-5-(phosphonooxy)pentyl]-5H,10H-benzo[g]pteridine-2,4-bis(olate) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | 7,8-dimethyl-10-[(2S,3S,4R)-2,3,4-trihydroxy-5-(phosphonooxy)pentyl]-5H-benzo[g]pteridine-2,4-bis(olate) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | [H][C@@](O)(COP(O)(O)=O)[C@@]([H])(O)[C@@]([H])(O)CN1C2=C(NC3=C1N=C([O-])N=C3[O-])C=C(C)C(C)=C2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as alkyldiarylamines. These are tertiary alkylarylamines having two aryl and one alkyl groups attached to the amino group. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organonitrogen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Amines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Tertiary amines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Alkyldiarylamines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Uracil + FMNH(2) + Oxygen > Ureidoacrylate peracid + Flavin Mononucleotide + Water Thymine + FMNH(2) + Oxygen > (Z)-2-Methyl-ureidoacrylate peracid + Flavin Mononucleotide + Water FMNH(2) + NAD > Flavin Mononucleotide + NADH An alkanesufonate (R-CH(2)-SO(3)H) + FMNH(2) + Oxygen > an aldehyde (R-CHO) + Flavin Mononucleotide + Sulfite + Water FMNH(2) + NADP > Flavin Mononucleotide + NADPH | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||