|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB003550 |
|---|
|
Identification |
|---|
| Name: |
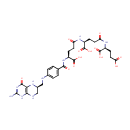
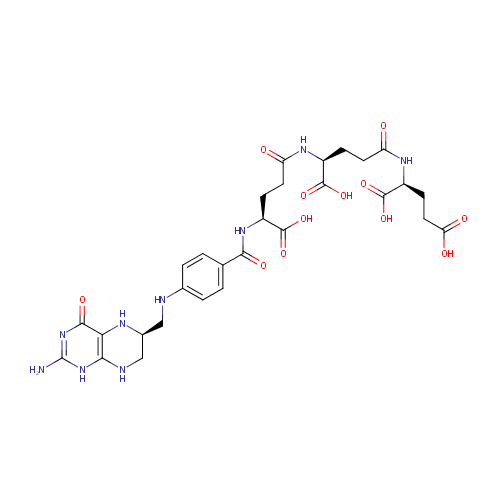
tetrahydropteroyltri-L-glutamate |
|---|
| Description: | Tetrahydropteroyltri-L-glutamate is an intermediate in the synthesis of methionine. It is a substrate for the enzyme 5-methyltetrahydropteroyltriglutamate--homocysteine methyltransferase which catalyzes the reaction 5-methyltetrahydropteroyltri-L-glutamate + L-homocysteine = tetrahydropteroyltri-L-glutamate + L-methionine. |
|---|
|
Structure |
|
|---|
| Synonyms: | - (2S)-2-(4S)-4-(4S)-4-4-(6S)-2-amino-4-oxo-5,6,7,8-tetrahydro-1H-Pteridin-6-ylmethylaminobenzoylamino-4-carboxybutanoylamino-4-carboxybutanoylaminopentanedioate
- (2S)-2-(4S)-4-(4S)-4-4-(6S)-2-amino-4-oxo-5,6,7,8-tetrahydro-1H-pteridin-6-ylmethylaminobenzoylamino-4-carboxybutanoylamino-4-carboxybutanoylaminopentanedioic acid
- N-4-({(6S)-2-amino-4-oxo-1,4,5,6,7,8-hexahydro-6-pteridinylmethyl}amino)benzoyl-L-g-glutamyl-L-g-glutamyl-L-glutamate
- N-4-({(6S)-2-amino-4-oxo-1,4,5,6,7,8-hexahydro-6-pteridinylmethyl}amino)benzoyl-L-g-glutamyl-L-g-glutamyl-L-glutamic acid
- N-4-({(6S)-2-amino-4-oxo-1,4,5,6,7,8-hexahydro-6-pteridinylmethyl}amino)benzoyl-L-gamma-glutamyl-L-gamma-glutamyl-L-glutamate
- N-4-({(6S)-2-Amino-4-oxo-1,4,5,6,7,8-hexahydro-6-pteridinylmethyl}amino)benzoyl-L-gamma-glutamyl-L-gamma-glutamyl-L-glutamic acid
- N-4-({(6S)-2-amino-4-oxo-1,4,5,6,7,8-hexahydro-6-pteridinylmethyl}amino)benzoyl-L-γ-glutamyl-L-γ-glutamyl-L-glutamate
- N-4-({(6S)-2-amino-4-oxo-1,4,5,6,7,8-hexahydro-6-pteridinylmethyl}amino)benzoyl-L-γ-glutamyl-L-γ-glutamyl-L-glutamic acid
- Tetrahydropteroyltri-L-glutamate
- Tetrahydropteroyltri-L-glutamic acid
|
|---|
|
Chemical Formula: |
C29H37N9O12 |
|---|
| Average Molecular Weight: |
703.6572 |
|---|
| Monoisotopic Molecular
Weight: |
703.256167693 |
|---|
| InChI Key: |
RXWVHRYZTWZATH-XSLAGTTESA-N |
|---|
| InChI: | InChI=1S/C29H37N9O12/c30-29-37-23-22(25(44)38-29)33-15(12-32-23)11-31-14-3-1-13(2-4-14)24(43)36-18(28(49)50)6-9-20(40)34-16(26(45)46)5-8-19(39)35-17(27(47)48)7-10-21(41)42/h1-4,15-18,31,33H,5-12H2,(H,34,40)(H,35,39)(H,36,43)(H,41,42)(H,45,46)(H,47,48)(H,49,50)(H4,30,32,37,38,44)/t15-,16-,17-,18-/m0/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (2S)-2-[(4S)-4-[(4S)-4-{[4-({[(6S)-2-amino-4-oxo-1,4,5,6,7,8-hexahydropteridin-6-yl]methyl}amino)phenyl]formamido}-4-carboxybutanamido]-4-carboxybutanamido]pentanedioic acid |
|---|
|
Traditional IUPAC Name: |
(2S)-2-[(4S)-4-[(4S)-4-{[4-({[(6S)-2-amino-4-oxo-5,6,7,8-tetrahydro-1H-pteridin-6-yl]methyl}amino)phenyl]formamido}-4-carboxybutanamido]-4-carboxybutanamido]pentanedioic acid |
|---|
| SMILES: | NC1=NC(=O)C2=C(NC[C@H](CNC3=CC=C(C=C3)C(=O)N[C@@H](CCC(=O)N[C@@H](CCC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)C(O)=O)N2)N1 |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as hippuric acids. These are compounds containing hippuric acid, which consists of a of a benzoyl group linked to the N-terminal of a glycine. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
|
Class |
Benzene and substituted derivatives |
|---|
| Sub Class | Benzamides |
|---|
|
Direct Parent |
Hippuric acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- N-acyl-aliphatic-alpha amino acid
- Tetracarboxylic acid or derivatives
- N-acyl-alpha amino acid or derivatives
- N-acyl-alpha-amino acid
- Hippuric acid
- Pterin
- Pteridine
- Aminobenzoic acid or derivatives
- Alpha-amino acid or derivatives
- N-substituted-alpha-amino acid
- Benzoic acid or derivatives
- Aminobenzamide
- Phenylalkylamine
- Substituted aniline
- Benzoyl
- Hydroxypyrimidine
- Secondary aliphatic/aromatic amine
- Aniline
- Amino fatty acid
- Fatty acyl
- Pyrimidine
- Heteroaromatic compound
- Secondary carboxylic acid amide
- Carboxamide group
- Azacycle
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Secondary amine
- Carboxylic acid
- Carboxylic acid derivative
- Carboximidic acid derivative
- Carboximidic acid
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|