|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB003528 |
|---|
|
Identification |
|---|
| Name: |
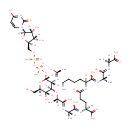
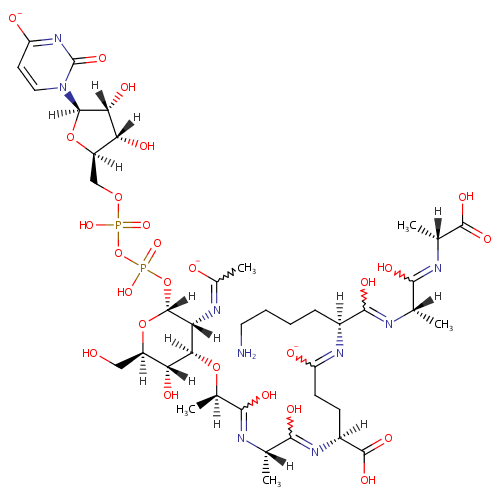
UDP-Mur2Ac(oyl-L-Ala-gamma-D-Glu-L-Lys-D-Ala-D-Ala) |
|---|
| Description: | UDP-Mur2Ac(oyl-L-Ala-gamma-D-Glu-L-Lys-D-Ala-D-Ala) is an intermediate in peptidoglycan biosynthesis. It is substrate for the enzyme Phospho-N-acetylmuramoyl-pentapeptide-transferase which catalyzes the first step of the lipid cycle reactions in the biosynthesis of the cell wall peptidoglycan. The reaction is UDP-Mur2Ac(oyl-L-Ala-gamma-D-Glu-L-Lys-D-Ala-D-Ala) + undecaprenyl phosphate = UMP + Mur2Ac(oyl-L-Ala-gamma-D-Glu-L-Lys-D-Ala-D-Ala)-diphosphoundecaprenol. |
|---|
|
Structure |
|
|---|
| Synonyms: | - UDP-Mur2Ac(oyl-L-Ala-g-D-Glu-L-Lys-D-Ala-D-Ala)
- UDP-Mur2ac(oyl-L-ala-γ-D-glu-L-lys-D-ala-D-ala)
- UDP-N-Acetylmuramoyl-L-alanyl-g-D-glutamyl-L-lysyl-D-alanyl-D-alanine
- UDP-N-acetylmuramoyl-L-alanyl-gamma-D-glutamyl-L-lysyl-D-alanyl-D-alanine
- UDP-N-Acetylmuramoyl-L-alanyl-γ-D-glutamyl-L-lysyl-D-alanyl-D-alanine
|
|---|
|
Chemical Formula: |
C40H62N9O26P2 |
|---|
| Average Molecular Weight: |
1146.9125 |
|---|
| Monoisotopic Molecular
Weight: |
1146.328121225 |
|---|
| InChI Key: |
PFMVORMCVGOQKR-MZSDELDXSA-K |
|---|
| InChI: | InChI=1S/C40H65N9O26P2/c1-16(32(57)44-18(3)37(61)62)43-35(60)21(8-6-7-12-41)46-25(52)10-9-22(38(63)64)47-33(58)17(2)42-34(59)19(4)71-31-27(45-20(5)51)39(73-23(14-50)29(31)55)74-77(68,69)75-76(66,67)70-15-24-28(54)30(56)36(72-24)49-13-11-26(53)48-40(49)65/h11,13,16-19,21-24,27-31,36,39,50,54-56H,6-10,12,14-15,41H2,1-5H3,(H,42,59)(H,43,60)(H,44,57)(H,45,51)(H,46,52)(H,47,58)(H,61,62)(H,63,64)(H,66,67)(H,68,69)(H,48,53,65)/p-3/t16-,17-,18+,19+,21-,22-,23+,24+,27+,28+,29+,30+,31+,36+,39+/m0/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (4S)-N-[(1S)-5-amino-1-{[(1S)-1-{[(1R)-1-carboxyethyl]-C-hydroxycarbonimidoyl}ethyl]-C-hydroxycarbonimidoyl}pentyl]-4-carboxy-4-{[(2S)-2-{[(2R)-2-{[(2R,3R,4R,5S,6R)-2-({[({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(4-oxido-2-oxo-1,2-dihydropyrimidin-1-yl)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)-5-hydroxy-6-(hydroxymethyl)-3-[(1-oxidoethylidene)amino]oxan-4-yl]oxy}-1-hydroxypropylidene]amino}-1-hydroxypropylidene]amino}butanecarboximidate |
|---|
|
Traditional IUPAC Name: |
(4S)-N-[(1S)-5-amino-1-{[(1S)-1-{[(1R)-1-carboxyethyl]-C-hydroxycarbonimidoyl}ethyl]-C-hydroxycarbonimidoyl}pentyl]-4-carboxy-4-{[(2S)-2-{[(2R)-2-{[(2R,3R,4R,5S,6R)-2-[({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(4-oxido-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy(hydroxy)phosphoryl}oxy(hydroxy)phosphoryl)oxy]-5-hydroxy-6-(hydroxymethyl)-3-[(1-oxidoethylidene)amino]oxan-4-yl]oxy}-1-hydroxypropylidene]amino}-1-hydroxypropylidene]amino}butanecarboximidate |
|---|
| SMILES: | [H][C@](C)(O[C@@]1([H])[C@]([H])(O)[C@@]([H])(CO)O[C@]([H])(OP(O)(=O)OP(O)(=O)OC[C@@]2([H])O[C@@]([H])(N3C=CC([O-])=NC3=O)[C@]([H])(O)[C@]2([H])O)[C@]1([H])N=C(C)[O-])C(O)=N[C@@]([H])(C)C(O)=N[C@@]([H])(CCC([O-])=N[C@@]([H])(CCCCN)C(O)=N[C@@]([H])(C)C(O)=N[C@]([H])(C)C(O)=O)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as pyrimidine nucleotide sugars. These are pyrimidine nucleotides bound to a saccharide derivative through the terminal phosphate group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
|
Class |
Pyrimidine nucleotides |
|---|
| Sub Class | Pyrimidine nucleotide sugars |
|---|
|
Direct Parent |
Pyrimidine nucleotide sugars |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pyrimidine nucleotide sugar
- Pyrimidine ribonucleoside diphosphate
- Alpha peptide
- N-acyl-alpha-hexosamine
- N-acyl-aliphatic-alpha amino acid
- N-acyl-alpha amino acid or derivatives
- N-acyl-alpha-amino acid
- Glucosamine
- Amino sugar
- N-glycosyl compound
- Glycosyl compound
- Organic pyrophosphate
- Monosaccharide phosphate
- Alpha-amino acid or derivatives
- Monoalkyl phosphate
- Amino saccharide
- Pyrimidone
- Alkyl phosphate
- Pyrimidine
- Phosphoric acid ester
- Oxane
- Organic phosphoric acid derivative
- Organic phosphate
- Monosaccharide
- Hydropyrimidine
- Dicarboxylic acid or derivatives
- Heteroaromatic compound
- Oxolane
- Secondary alcohol
- 1,2-diol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Ether
- Dialkyl ether
- Carboxylic acid
- Carboxylic acid derivative
- Carboximidic acid derivative
- Carboximidic acid
- Hydrocarbon derivative
- Primary amine
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Amine
- Alcohol
- Organic anion
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -3 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | Not Available | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|