|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB003516 |
|---|
|
Identification |
|---|
| Name: |
2-Methyl-3-oxopropanoate |
|---|
| Description: | 2-Methyl-3-oxopropanoic acid is an intermediate in the metabolism of Propanoate. It is a substrate for 3-hydroxyisobutyrate dehydrogenase, Alanine--glyoxylate aminotransferase and Methylmalonate-semialdehyde dehydrogenase. |
|---|
|
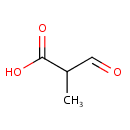
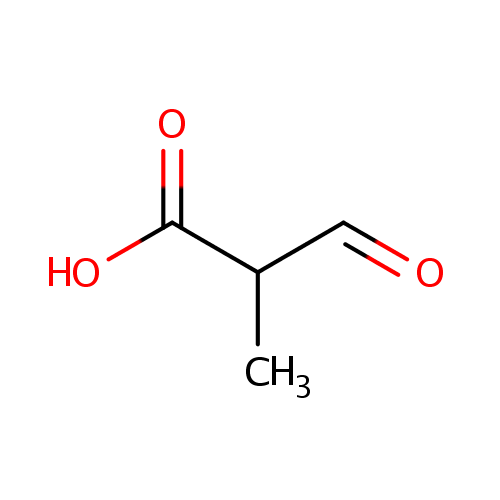
Structure |
|
|---|
| Synonyms: | - (S)-2-methyl-3-oxopropanoate
- (S)-ch3-malonate-semialdehyde
- (s)-2-Methyl-3-oxopropanoate
- (s)-2-Methyl-3-oxopropanoic acid
- (s)-CH3-Malonate-semialdehyde
- (s)-CH3-Malonic acid-semialdehyde
- 2-Methyl-3-oxopropanoic acid
- 3-oxo-2-methylpropanoate
- 3-Oxo-2-methylpropanoic acid
- Ch3-malonate-semialdehyde
- CH3-Malonic acid-semialdehyde
- Methylmalonate semialdehyde
- Methylmalonate-semialdehyde
- Methylmalonic acid semialdehyde
- Methylmalonic acid-semialdehyde
|
|---|
|
Chemical Formula: |
C4H6O3 |
|---|
| Average Molecular Weight: |
102.0886 |
|---|
| Monoisotopic Molecular
Weight: |
102.031694058 |
|---|
| InChI Key: |
VOKUMXABRRXHAR-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C4H6O3/c1-3(2-5)4(6)7/h2-3H,1H3,(H,6,7) |
|---|
| CAS
number: |
6236-08-4 |
|---|
| IUPAC Name: | 2-methyl-3-oxopropanoic acid |
|---|
|
Traditional IUPAC Name: |
methylmalonate semialdehyde |
|---|
| SMILES: | CC(C=O)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as 1,3-dicarbonyl compounds. These are carbonyl compounds with the generic formula O=C(R)C(H)C(R')=O, where R and R' can be any group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organooxygen compounds |
|---|
|
Class |
Carbonyl compounds |
|---|
| Sub Class | 1,3-dicarbonyl compounds |
|---|
|
Direct Parent |
1,3-dicarbonyl compounds |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 1,3-dicarbonyl compound
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Short-chain aldehyde
- Aldehyde
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Robinson, Wm. G.; Coon, Minor J. Purification and properties of b-hydroxyisobutyric dehydrogenase. Journal of Biological Chemistry (1957), 225 511-21. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|