|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB003481 |
|---|
|
Identification |
|---|
| Name: |
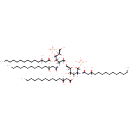
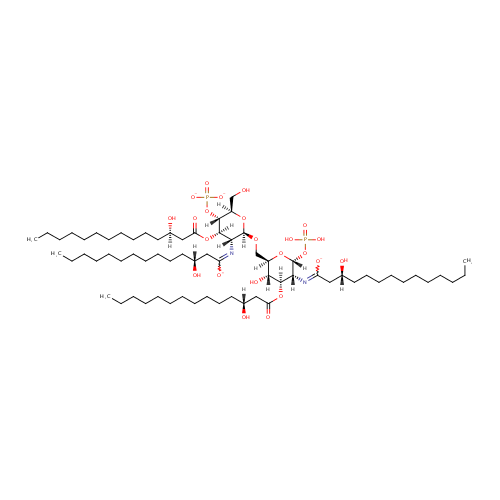
(2-N,3-O-bis(3-Hydroxytetradecanoyl)-4-O-phosphono-beta-D-glucosaminyl)-(1->6)-(2-N,3-O-bis(3-hydroxytetradecanoyl)-beta-D-glucosaminyl phosphate) |
|---|
| Description: | (2-N,3-O-bis(3-hydroxytetradecanoyl)-4-O-phosphono-beta-D-glucosaminyl)-(1->6)-(2-N,3-O-bis(3-hydroxytetradecanoyl)-beta-D-glucosaminyl phosphate) is a substrate for tetraacyldisaccharide 4'-kinase. This is an enzyme that phosphorylates the 4'-position of a tetraacyldisaccharide 1-phosphate precursor (DS-1-P) of lipopolysaccharide lipid A. This lipid which is part of LPS, forms the outer membranes of Gram-negative bacteria. This enzyme catalyzes the chemical reaction: ATP + [2-N,3-O-bis(3-hydroxytetradecanoyl)-beta-D-glucosaminyl]-(1->6)-[2- N,3-O-bis(3-hydroxytetradecanoyl)-beta-D-glucosaminyl phosphate] <=> ADP + [2-N,3-O-bis(3-hydroxytetradecanoyl)-4-O-phosphono-beta-D-glucosaminyl]-(1->6)-[2-N,3-O-bis(3-hydroxytetradecanoyl)-beta-D- glucosaminyl phosphate] |
|---|
|
Structure |
|
|---|
| Synonyms: | - (2-N,3-O-Bis(3-hydroxytetradecanoyl)-4-O-phosphono-b-D-glucosaminyl)-(1->6)-(2-N,3-O-bis(3-hydroxytetradecanoyl)-b-D-glucosaminyl phosphate)
- (2-N,3-O-Bis(3-hydroxytetradecanoyl)-4-O-phosphono-b-D-glucosaminyl)-(1->6)-(2-N,3-O-bis(3-hydroxytetradecanoyl)-b-D-glucosaminyl phosphoric acid)
- (2-N,3-O-Bis(3-hydroxytetradecanoyl)-4-O-phosphono-beta-D-glucosaminyl)-(1->6)-(2-N,3-O-bis(3-hydroxytetradecanoyl)-beta-D-glucosaminyl phosphoric acid)
- (2-N,3-O-Bis(3-hydroxytetradecanoyl)-4-O-phosphono-β-D-glucosaminyl)-(1->6)-(2-N,3-O-bis(3-hydroxytetradecanoyl)-β-D-glucosaminyl phosphate)
- (2-N,3-O-Bis(3-hydroxytetradecanoyl)-4-O-phosphono-β-D-glucosaminyl)-(1->6)-(2-N,3-O-bis(3-hydroxytetradecanoyl)-β-D-glucosaminyl phosphoric acid)
- (2R,3R,4R,5S,6R)-5-hydroxy-6-(2R,3R,4R,5S,6R)-6-(hydroxymethyl)-3-(3R)-3-hydroxytetradecanoylamino-4-(3R)-3-hydroxytetradecanoyloxy-5-phosphonatooxyoxan-2-yloxymethyl-3-(3R)-3-hydroxytetradecanoylamino-4-(3R)-3-hydroxytetradecanoyloxyoxan-2-yl phosphate
- (2R,3R,4R,5S,6R)-5-Hydroxy-6-(2R,3R,4R,5S,6R)-6-(hydroxymethyl)-3-(3R)-3-hydroxytetradecanoylamino-4-(3R)-3-hydroxytetradecanoyloxy-5-phosphonatooxyoxan-2-yloxymethyl-3-(3R)-3-hydroxytetradecanoylamino-4-(3R)-3-hydroxytetradecanoyloxyoxan-2-yl phosphoric acid
- 2-Deoxy-6-O-(2-deoxy-3-O-(3R)-3-hydroxytetradecanoyl-2-{(3R)-3-hydroxytetradecanoylamino}-4-O-phosphonato-b-D-glucopyranosyl)-3-O-(3R)-3-hydroxytetradecanoyl-2-{(3R)-3-hydroxytetradecanoylamino}-1-O-phosphonato-a-D-glucopyranose
- 2-Deoxy-6-O-(2-deoxy-3-O-(3R)-3-hydroxytetradecanoyl-2-{(3R)-3-hydroxytetradecanoylamino}-4-O-phosphonato-beta-D-glucopyranosyl)-3-O-(3R)-3-hydroxytetradecanoyl-2-{(3R)-3-hydroxytetradecanoylamino}-1-O-phosphonato-alpha-D-glucopyranose
- 2-Deoxy-6-O-(2-deoxy-3-O-(3R)-3-hydroxytetradecanoyl-2-{(3R)-3-hydroxytetradecanoylamino}-4-O-phosphonato-β-D-glucopyranosyl)-3-O-(3R)-3-hydroxytetradecanoyl-2-{(3R)-3-hydroxytetradecanoylamino}-1-O-phosphonato-α-D-glucopyranose
|
|---|
|
Chemical Formula: |
C68H126N2O23P2 |
|---|
| Average Molecular Weight: |
1401.6752 |
|---|
| Monoisotopic Molecular
Weight: |
1400.822661372 |
|---|
| InChI Key: |
KVJWZTLXIROHIL-QDORLFPLSA-J |
|---|
| InChI: | InChI=1S/C68H130N2O23P2/c1-5-9-13-17-21-25-29-33-37-41-51(72)45-57(76)69-61-65(90-59(78)47-53(74)43-39-35-31-27-23-19-15-11-7-3)63(80)56(89-68(61)93-95(84,85)86)50-87-67-62(70-58(77)46-52(73)42-38-34-30-26-22-18-14-10-6-2)66(64(55(49-71)88-67)92-94(81,82)83)91-60(79)48-54(75)44-40-36-32-28-24-20-16-12-8-4/h51-56,61-68,71-75,80H,5-50H2,1-4H3,(H,69,76)(H,70,77)(H2,81,82,83)(H2,84,85,86)/p-4/t51-,52-,53-,54-,55-,56-,61-,62-,63-,64-,65-,66-,67-,68-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (3R)-3-hydroxy-N-[(2R,3R,4R,5S,6R)-5-hydroxy-6-({[(2R,3R,4R,5S,6R)-3-{[(3R)-3-hydroxy-1-oxidotetradecylidene]amino}-6-(hydroxymethyl)-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-5-(phosphonatooxy)oxan-2-yl]oxy}methyl)-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-2-(phosphonooxy)oxan-3-yl]tetradecanecarboximidate |
|---|
|
Traditional IUPAC Name: |
(3R)-3-hydroxy-N-[(2R,3R,4R,5S,6R)-5-hydroxy-6-({[(2R,3R,4R,5S,6R)-3-{[(3R)-3-hydroxy-1-oxidotetradecylidene]amino}-6-(hydroxymethyl)-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-5-(phosphonatooxy)oxan-2-yl]oxy}methyl)-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-2-(phosphonooxy)oxan-3-yl]tetradecanecarboximidate |
|---|
| SMILES: | [H][C@@](O)(CCCCCCCCCCC)CC(=O)O[C@@]1([H])[C@]([H])(O)[C@@]([H])(CO[C@]2([H])O[C@]([H])(CO)[C@@]([H])(OP([O-])([O-])=O)[C@]([H])(OC(=O)C[C@]([H])(O)CCCCCCCCCCC)[C@@]2([H])N=C([O-])C[C@]([H])(O)CCCCCCCCCCC)O[C@]([H])(OP(O)(O)=O)[C@]1([H])N=C([O-])C[C@]([H])(O)CCCCCCCCCCC |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as saccharolipids. These are compounds in which fatty acids are linked directly to a sugar backbone, forming structures that are compatible with membrane bilayers. In the saccharolipids, a sugar substitutes for the glycerol backbone that is present in glycerolipids and glycerophospholipids. The most familiar saccharolipids contain an acylated glucosamine. In contrast to others glycolipids, the fatty acid is not glycosidically linked to the sugar moiety. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Saccharolipids |
|---|
| Sub Class | Not Available |
|---|
|
Direct Parent |
Saccharolipids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Saccharolipid
- N-acyl-alpha-hexosamine
- Disaccharide phosphate
- Glucosamine
- Amino sugar
- O-glycosyl compound
- Glycosyl compound
- Disaccharide
- Monoalkyl phosphate
- Amino saccharide
- Fatty acid ester
- Beta-hydroxy acid
- Fatty acyl
- Alkyl phosphate
- Phosphoric acid ester
- Oxane
- Organic phosphoric acid derivative
- Organic phosphate
- Hydroxy acid
- Dicarboxylic acid or derivatives
- Saccharide
- Secondary alcohol
- Carboxylic acid ester
- Oxacycle
- Organoheterocyclic compound
- Carboxylic acid derivative
- Carboximidic acid derivative
- Carboximidic acid
- Acetal
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Alcohol
- Organic anion
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Membrane |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Lipopolysaccharide biosynthesis pae00540
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | 58603 | | HMDB ID | Not Available | | Pubchem Compound ID | 10329123 | | Kegg ID | Not Available | | ChemSpider ID | 8504584 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|