|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB003432 |
|---|
|
Identification |
|---|
| Name: |
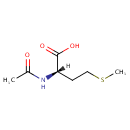
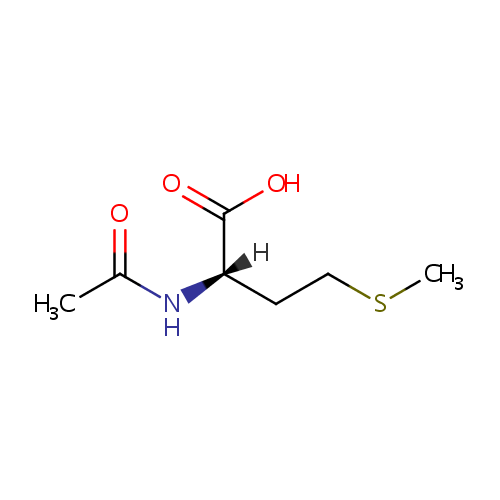
N-Acetyl-L-methionine |
|---|
| Description: | N-Acetyl-L-methionine is an acylated methionine derivative. The N-terminal methionine is often released from peptides and proteins in Pseudomonas aeruginosa via methionine aminopeptidase. The free methionine can be acetylated by the enzyme YncA or N acyltransferase in a reaction using a CoA thioester as cofactor. |
|---|
|
Structure |
|
|---|
| Synonyms: | - (2S)-2-acetamido-4-Methylsulfanylbutanoate
- (2S)-2-acetamido-4-methylsulfanylbutanoic acid
- (2S)-2-acetamido-4-Methylsulphanylbutanoate
- (2S)-2-acetamido-4-Methylsulphanylbutanoic acid
- Acetyl-l-methionine
- Acetylmethionin
- Acetylmethionine
- DL-n-acetylmethionine
- L-(n-acetyl)methionine
- L-n-acetyl-Methionine
- Methionamine
- Methionin
- N-acetyl(methyl)homocysteine
- N-acetyl-Methionine
- N-acetyl-S-methylhomocysteine
- N-acetylmethionine
- Thiomedon
|
|---|
|
Chemical Formula: |
C7H13NO3S |
|---|
| Average Molecular Weight: |
191.248 |
|---|
| Monoisotopic Molecular
Weight: |
191.061613977 |
|---|
| InChI Key: |
XUYPXLNMDZIRQH-LURJTMIESA-N |
|---|
| InChI: | InChI=1S/C7H13NO3S/c1-5(9)8-6(7(10)11)3-4-12-2/h6H,3-4H2,1-2H3,(H,8,9)(H,10,11)/t6-/m0/s1 |
|---|
| CAS
number: |
65-82-7 |
|---|
| IUPAC Name: | (2S)-2-acetamido-4-(methylsulfanyl)butanoic acid |
|---|
|
Traditional IUPAC Name: |
N-acetylmethionine |
|---|
| SMILES: | [H][C@@](CCSC)(NC(C)=O)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as n-acyl-aliphatic-alpha amino acids. These are alpha amino acids carrying a N-acylated aliphatic chain. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
|
Direct Parent |
N-acyl-aliphatic-alpha amino acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- N-acyl-aliphatic-alpha amino acid
- N-acyl-l-alpha-amino acid
- Methionine or derivatives
- Thia fatty acid
- Amino fatty acid
- Fatty acyl
- Fatty acid
- Acetamide
- Secondary carboxylic acid amide
- Carboxamide group
- Dialkylthioether
- Sulfenyl compound
- Thioether
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid amide
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
105.5 C |
|---|
| Experimental Properties: |
| Property | Value | Source |
|---|
| Water Solubility: | 307mg/mL at 25oC [BEILSTEIN] | PhysProp |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|