|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB003416 |
|---|
|
Identification |
|---|
| Name: |
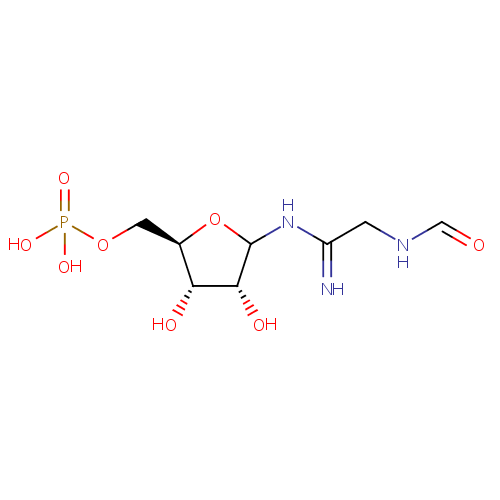
2-(Formamido)-N(1)-(5-phospho-D-ribosyl)acetamidine |
|---|
| Description: | 2-(Formamido)-N1-(5-phospho-D-ribosyl)acetamidine is an intermediate in purine metabolism. The enzyme phosphoribosylformylglycinamidine synthase [EC:6.3.5.3] catalyzes the production of this metabolite from N2-formyl-N1-(5-phospho-D-ribosyl)glycinamide. |
|---|
|
Structure |
|
|---|
| Synonyms: | - (2R,3S,4R)-5-(Z)-(1-amino-2-formamidoethylidene)amino-3,4-dihydroxyoxolan-2-ylmethyl dihydrogen phosphate
- (2R,3S,4R)-5-(Z)-(1-amino-2-Formamidoethylidene)amino-3,4-dihydroxyoxolan-2-ylmethyl dihydrogen phosphoric acid
- 1-(5'-Phosphoribosyl)-N-formylglycinamidine
- 1-deoxy-1-[2-(formamido)acetimidamido]-D-ribofuranose 5-(dihydrogen phosphate)
- 1-Deoxy-1-[2-(formamido)acetimidamido]-D-ribofuranose 5-(dihydrogen phosphoric acid)
- 2-(formamido)-N(1)-(5'-phosphoribosyl)acetamidine
- 2-(Formamido)-N1-(5'-phosphoribosyl)acetamidine
- 2-(Formamido)-N1-(5-phospho-D-ribosyl)acetamidine
- 5'-phosphoribosyl-N-formyl glycineamidine
- 5'-Phosphoribosyl-n-formyl glycineamidine
- 5'-Phosphoribosyl-N-formylglycinamidine
- 5'-Phosphoribosylformylglycinamidine
- FGAM
- N-(2-Formamidoethanimidoyl)-5-O-phosphono-D-ribofuranosylamine
- N-[2-(formamido)ethanimidoyl]-5-O-phosphono-D-ribofuranosylamine
- [(2R,3S,4R,5R)-5-[(1-amino-2-formamido-Ethylidene)amino]-3,4-dihydroxy-oxolan-2-yl]methoxyphosphonate
- [(2R,3S,4R,5R)-5-[(1-amino-2-formamido-ethylidene)amino]-3,4-dihydroxy-oxolan-2-yl]methoxyphosphonic acid
|
|---|
|
Chemical Formula: |
C8H16N3O8P |
|---|
| Average Molecular Weight: |
313.2017 |
|---|
| Monoisotopic Molecular
Weight: |
313.067501015 |
|---|
| InChI Key: |
PMCOGCVKOAOZQM-ZRTZXPPTSA-N |
|---|
| InChI: | InChI=1S/C8H16N3O8P/c9-5(1-10-3-12)11-8-7(14)6(13)4(19-8)2-18-20(15,16)17/h3-4,6-8,13-14H,1-2H2,(H2,9,11)(H,10,12)(H2,15,16,17)/t4-,6-,7-,8?/m1/s1 |
|---|
| CAS
number: |
6157-85-3 |
|---|
| IUPAC Name: | {[(2R,3S,4R)-3,4-dihydroxy-5-(2-formamidoethanimidamido)oxolan-2-yl]methoxy}phosphonic acid |
|---|
|
Traditional IUPAC Name: |
FGAM |
|---|
| SMILES: | O[C@H]1[C@@H](O)C(NC(=N)CNC=O)O[C@@H]1COP(O)(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as glycosylamines. These are compounds consisting of an?amine?with a?beta-N-glycosidic bond?to a carbohydrate, thus forming a cyclic?hemiaminal ether?bond (alpha-amino ether). |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organooxygen compounds |
|---|
|
Class |
Carbohydrates and carbohydrate conjugates |
|---|
| Sub Class | Glycosyl compounds |
|---|
|
Direct Parent |
Glycosylamines |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- N-glycosyl compound
- Monosaccharide phosphate
- Monoalkyl phosphate
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic phosphate
- Monosaccharide
- Oxolane
- Secondary alcohol
- 1,2-diol
- Oxacycle
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboximidamide
- Carboximidic acid derivative
- Carboximidic acid
- Carboxylic acid amidine
- Amidine
- Hydrocarbon derivative
- Organonitrogen compound
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|