|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001829 |
|---|
|
Identification |
|---|
| Name: |
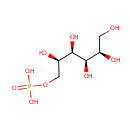
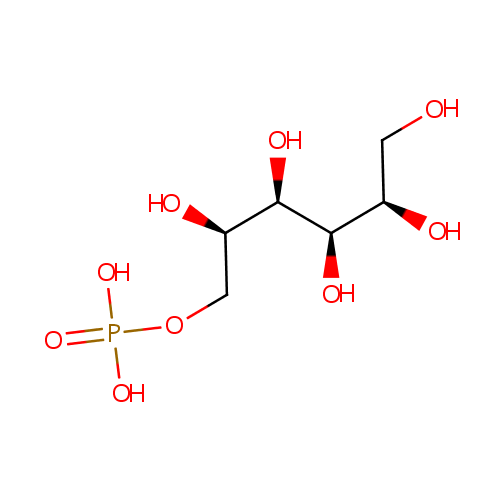
Mannitol 1-phosphate |
|---|
| Description: | Mannitol-1-phosphate is a sugar alcohol. Mannitol-1-phosphate dehydrogenase (EC 1.1.1.17) reduces fructose 6-phosphate into mannitol 1-phosphate, in the mannitol cycle of organisms such as Lactobacillus plantarum, a lactic acid bacterium found in many fermented food products and in the gastrointestinal tract of mammals. (HMDB) This redox reaction also occurs in Pseudomonas aeruginosa, catalyzed by mannitol-1-phosphate 5-dehydrogenase which is encoded by gene mtlD. (EcoCyc) In Pseudomonas aeruginosa, the transport of mannitol into the cell is done through a phosphotransferase system which attaches a phosphate to mannitol, producing mannitol 1-phosphate. (KEGG) |
|---|
|
Structure |
|
|---|
| Synonyms: | - D-Mannitol-1-phosphate
- D-Mannitol-1-phosphoric acid
- D-Mannitol-6-phosphate
- D-Mannitol-6-phosphoric acid
- Mannitol 1-phosphoric acid
- Mannitol-1-P
- Mannitol-1-phosphate
- Mannitol-1-phosphoric acid
- Mannitol-6-P
- Mant1P
|
|---|
|
Chemical Formula: |
C6H15O9P |
|---|
| Average Molecular Weight: |
262.1517 |
|---|
| Monoisotopic Molecular
Weight: |
262.04536859 |
|---|
| InChI Key: |
GACTWZZMVMUKNG-KVTDHHQDSA-N |
|---|
| InChI: | InChI=1S/C6H15O9P/c7-1-3(8)5(10)6(11)4(9)2-15-16(12,13)14/h3-11H,1-2H2,(H2,12,13,14)/t3-,4-,5-,6-/m1/s1 |
|---|
| CAS
number: |
15806-48-1 |
|---|
| IUPAC Name: | {[(2R,3R,4R,5R)-2,3,4,5,6-pentahydroxyhexyl]oxy}phosphonic acid |
|---|
|
Traditional IUPAC Name: |
mannitol-1-phosphate |
|---|
| SMILES: | OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)COP(O)(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as monoalkyl phosphates. These are organic compounds containing a phosphate group that is linked to exactly one alkyl chain. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organophosphorus compounds |
|---|
|
Class |
Organic phosphoric acids and derivatives |
|---|
| Sub Class | Phosphate esters |
|---|
|
Direct Parent |
Monoalkyl phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Monoalkyl phosphate
- Organic phosphate
- Secondary alcohol
- Polyol
- 1,2-diol
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Fructose and mannose metabolism pae00051
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|