|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001782 |
|---|
|
Identification |
|---|
| Name: |
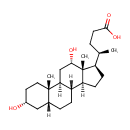
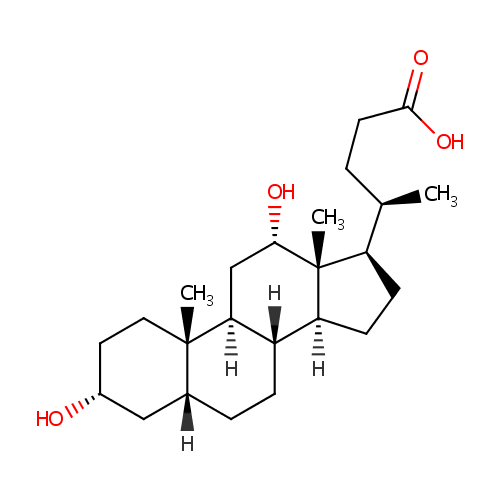
Deoxycholic acid |
|---|
| Description: | Deoxycholic acid is a bile acid formed by bacterial action from cholate. It is usually conjugated with glycine or taurine. Deoxycholic acid acts as a detergent to solubilize fats for intestinal absorption, is reabsorbed itself, and is used as a choleretic and detergent. Bile acids are steroid acids found predominantly in bile of mammals. The distinction between different bile acids is minute, depends only on presence or absence of hydroxyl groups on positions 3, 7, and 12. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 3α,12α-dihydroxy-5β-cholanate
- 3α,12α-dihydroxy-5β-cholanic acid
- 3a,12a-Dihydroxy-5b-cholanate
- 3a,12a-Dihydroxy-5b-cholanic acid
- 3alpha,12alpha-dihydroxy-5beta-cholanate
- 3alpha,12alpha-dihydroxy-5beta-cholanic acid
- 3α,12α-Dihydroxy-5β-cholanate
- 3α,12α-Dihydroxy-5β-cholanic acid
- 5b-Cholanate-3a,12a-diol
- 5b-Cholanic acid-3a,12a-diol
- 5b-Deoxycholate
- 5b-Deoxycholic acid
- 7-Deoxycholate
- 7-Deoxycholic acid
- Cholerebic
- Cholorebic
- Degalol
- Deoxy-Cholate
- Deoxy-Cholic acid
- Deoxycholatate
- Deoxycholate
- Deoxycholatic acid
- Deoxycholic acid
|
|---|
|
Chemical Formula: |
C24H40O4 |
|---|
| Average Molecular Weight: |
392.572 |
|---|
| Monoisotopic Molecular
Weight: |
392.292659768 |
|---|
| InChI Key: |
KXGVEGMKQFWNSR-LLQZFEROSA-N |
|---|
| InChI: | InChI=1S/C24H40O4/c1-14(4-9-22(27)28)18-7-8-19-17-6-5-15-12-16(25)10-11-23(15,2)20(17)13-21(26)24(18,19)3/h14-21,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15-,16-,17+,18-,19+,20+,21+,23+,24-/m1/s1 |
|---|
| CAS
number: |
83-44-3 |
|---|
| IUPAC Name: | (4R)-4-[(1S,2S,5R,7R,10R,11S,14R,15R,16S)-5,16-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0?,??0??,???heptadecan-14-yl]pentanoic acid |
|---|
|
Traditional IUPAC Name: |
deoxycholic acid |
|---|
| SMILES: | [H][C@@]12CC[C@H]([C@H](C)CCC(O)=O)[C@@]1(C)[C@@H](O)C[C@@]1([H])[C@@]2([H])CC[C@]2([H])C[C@H](O)CC[C@]12C |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as dihydroxy bile acids, alcohols and derivatives. These are compounds containing or derived from a bile acid or alcohol, and which bears exactly two carboxylic acid groups. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
|
Direct Parent |
Dihydroxy bile acids, alcohols and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Dihydroxy bile acid, alcohol, or derivatives
- 3-alpha-hydroxysteroid
- Hydroxysteroid
- 12-hydroxysteroid
- 3-hydroxysteroid
- Cyclic alcohol
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework |
Aliphatic homopolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
171-174 °C |
|---|
| Experimental Properties: |
| Property | Value | Source |
|---|
| Water Solubility: | 0.0436 mg/mL [YALKOWSKY,SH & DANNENFELSER,RM (1992)] | PhysProp | | LogP: | 3.50 [RODA,A ET AL. (1990)] | PhysProp |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Membrane |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Andersen RB, Bruusgaard A: Effect of the common bile acids on the fibrin/fibrinogen fragments in rheumatoid synovial fluid. A possible clue to the ameliorating effect of jaundice in rheumatoid arthritis. Scand J Rheumatol. 1975;4(3):158-64. Pubmed: 52191
- Beher WT, Gabbard A, Norum RA, Stradnieks S: Effect of blood high density lipoprotein cholesterol concentration on fecal steroid excretion in humans. Life Sci. 1983 Jun 27;32(26):2933-7. Pubmed: 6865641
- Berr F, Stellaard F, Pratschke E, Paumgartner G: Effects of cholecystectomy on the kinetics of primary and secondary bile acids. J Clin Invest. 1989 May;83(5):1541-50. Pubmed: 2708522
- Chiang JY: Bile acid regulation of hepatic physiology: III. Bile acids and nuclear receptors. Am J Physiol Gastrointest Liver Physiol. 2003 Mar;284(3):G349-56. Pubmed: 12576301
- Claudel T, Staels B, Kuipers F: The Farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol. 2005 Oct;25(10):2020-30. Epub 2005 Jul 21. Pubmed: 16037564
- Costarelli V, Sanders TA: Plasma deoxycholic acid concentration is elevated in postmenopausal women with newly diagnosed breast cancer. Eur J Clin Nutr. 2002 Sep;56(9):925-7. Pubmed: 12209383
- Davis RA, Miyake JH, Hui TY, Spann NJ: Regulation of cholesterol-7alpha-hydroxylase: BAREly missing a SHP. J Lipid Res. 2002 Apr;43(4):533-43. Pubmed: 11907135
- Deleze G, Paumgartner G, Karlaganis G, Giger W, Reinhard M, Sidiropoulos D: Bile acid pattern in human amniotic fluid. Eur J Clin Invest. 1978 Feb;8(1):41-5. Pubmed: 417931
- Duret G, Delcour AH: Deoxycholic acid blocks vibrio cholerae OmpT but not OmpU porin. J Biol Chem. 2006 Jul 21;281(29):19899-905. Epub 2006 May 2. Pubmed: 16670088
- Heikkinen J, Maentausta O, Tuimala R, Ylostalo P, Janne O: Amniotic fluid bile acids in normal and pathologic pregnancy. Obstet Gynecol. 1980 Jul;56(1):60-4. Pubmed: 7383489
- Nobuoka A, Takayama T, Miyanishi K, Sato T, Takanashi K, Hayashi T, Kukitsu T, Sato Y, Takahashi M, Okamoto T, Matsunaga T, Kato J, Oda M, Azuma T, Niitsu Y: Glutathione-S-transferase P1-1 protects aberrant crypt foci from apoptosis induced by deoxycholic acid. Gastroenterology. 2004 Aug;127(2):428-43. Pubmed: 15300575
- Rudi J, Schonig T, Stremmel W: -Therapy with ursodeoxycholic acid in primary biliary cirrhosis in pregnancy-. Z Gastroenterol. 1996 Mar;34(3):188-91. Pubmed: 8650973
- Salen G, Tint GS, Eliav B, Deering N, Mosbach EH: Increased formation of ursodeoxycholic acid in patients treated with chenodeoxycholic acid. J Clin Invest. 1974 Feb;53(2):612-21. Pubmed: 11344576
- St-Pierre MV, Kullak-Ublick GA, Hagenbuch B, Meier PJ: Transport of bile acids in hepatic and non-hepatic tissues. J Exp Biol. 2001 May;204(Pt 10):1673-86. Pubmed: 11316487
- Stadler J, Yeung KS, Furrer R, Marcon N, Himal HS, Bruce WR: Proliferative activity of rectal mucosa and soluble fecal bile acids in patients with normal colons and in patients with colonic polyps or cancer. Cancer Lett. 1988 Jan;38(3):315-20. Pubmed: 3349450
- Stellard F, Paumgartner G, van Berge Henegouwen GP, van der Werf SD: Determination of deoxycholic acid pool size and input rate using [24-13C]deoxycholic acid and serum sampling. J Lipid Res. 1986 Nov;27(11):1222-5. Pubmed: 3559388
- Tadano T, Kanoh M, Matsumoto M, Sakamoto K, Kamano T: Studies of serum and feces bile acids determination by gas chromatography-mass spectrometry. Rinsho Byori. 2006 Feb;54(2):103-10. Pubmed: 16548228
- Yamaga N, Adachi K, Shimizu K, Miyake S, Sumi F, Miyagawa I, Goto H: Bile acids of patients with renal failure receiving chronic hemodialysis. Steroids. 1986 Nov-Dec;48(5-6):427-38. Pubmed: 3445292
|
|---|
| Synthesis Reference: |
He, Zhiquan. in-situ generation of deoxycholic acid by poultry for Chinese medicine. Faming Zhuanli Shenqing Gongkai Shuomingshu (1993), 3 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|