|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001731 |
|---|
|
Identification |
|---|
| Name: |
Ethanesulfonate |
|---|
| Description: | Ethanesulfonate is a member of the chemical class known as Depsipeptides. These are natural or synthetic compounds having sequences of amino and hydroxy carboxylic acid residues (usually -amino and -hydroxy acids), commonly but not necessarily regularly alternating. It is also called Coenzyme M. Coenzyme M is a coenzyme required for methyl-transfer reactions in the metabolism of methanogens. The coenzyme is an anion with the formula HSCH2CH2SO_3. It is named 2-mercaptoethanesulfonate and abbreviated HS |
|---|
|
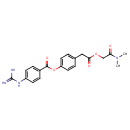
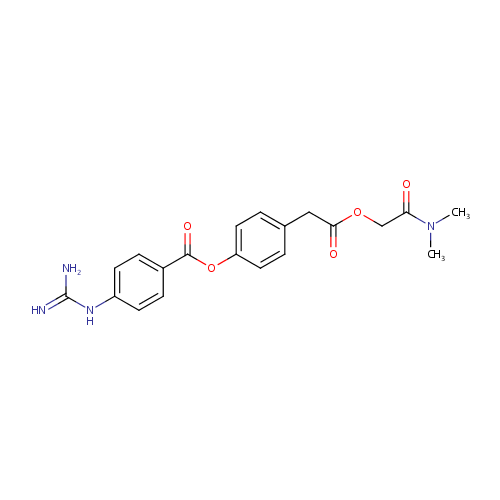
Structure |
|
|---|
| Synonyms: | - 1-THIOETHANEsulfonate
- 1-THIOETHANESULFONIC ACID
- 1-THIOETHANEsulphonate
- 1-THIOETHANEsulphonic acid
- 2-Mercaptoethanesulfonate
- 2-Mercaptoethanesulfonic acid
- 2-Mercaptoethanesulphonate
- 2-Mercaptoethanesulphonic acid
- 2-Mercaptoethylsulfonate
- 2-Mercaptoethylsulfonic acid
- 2-Mercaptoethylsulphonate
- 2-Mercaptoethylsulphonic acid
- 2-Sulfanylethanesulfonate
- 2-Sulfanylethanesulfonic acid
- 2-Sulfanylethylsulfonate
- 2-Sulfanylethylsulfonic acid
- 2-Sulphanylethanesulphonate
- 2-Sulphanylethanesulphonic acid
- 2-Sulphanylethylsulphonate
- 2-Sulphanylethylsulphonic acid
- b-Mercaptoethanesulfonate
- b-Mercaptoethanesulfonic acid
- b-Mercaptoethanesulphonate
- b-Mercaptoethanesulphonic acid
- Beta-Mercaptoethanesulfonate
- Beta-Mercaptoethanesulfonic acid
- Beta-Mercaptoethanesulphonate
- Beta-Mercaptoethanesulphonic acid
- Camostat mesilate
- Camostat mesilic acid
- Camostat mesylate
- Camostat mesylic acid
- Camostat monomethanesulfonate
- Camostat monomethanesulfonic acid
- Camostat monomethanesulphonate
- Camostat monomethanesulphonic acid
- Coenzima M
- Coenzym M
- Coenzyme M
- CoM
- Ethanesulfonic acid
- Ethanesulphonate
- Ethanesulphonic acid
- HS-CoM
- Reduced coenzyme M
- Reduced CoM
- β-Mercaptoethanesulfonate
- β-Mercaptoethanesulfonic acid
- β-Mercaptoethanesulphonate
- β-Mercaptoethanesulphonic acid
|
|---|
|
Chemical Formula: |
C20H22N4O5 |
|---|
| Average Molecular Weight: |
398.4125 |
|---|
| Monoisotopic Molecular
Weight: |
398.159019834 |
|---|
| InChI Key: |
XASIMHXSUQUHLV-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C20H22N4O5/c1-24(2)17(25)12-28-18(26)11-13-3-9-16(10-4-13)29-19(27)14-5-7-15(8-6-14)23-20(21)22/h3-10H,11-12H2,1-2H3,(H4,21,22,23) |
|---|
| CAS
number: |
59721-29-8 |
|---|
| IUPAC Name: | 4-{2-[(dimethylcarbamoyl)methoxy]-2-oxoethyl}phenyl 4-carbamimidamidobenzoate |
|---|
|
Traditional IUPAC Name: |
camostat |
|---|
| SMILES: | CN(C)C(=O)COC(=O)CC1=CC=C(OC(=O)C2=CC=C(NC(N)=N)C=C2)C=C1 |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as depsides and depsidones. These are polycyclic compounds that is either a polyphenolic compound composed of two or more monocyclic aromatic units linked by an ester bond (depside), or a compound containing the depsidone structure (depsidone). |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
|
Class |
Depsides and depsidones |
|---|
| Sub Class | Not Available |
|---|
|
Direct Parent |
Depsides and depsidones |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Depside backbone
- Guanidinobenzoic acid or derivatives
- Phenylacetate
- Phenol ester
- Benzoate ester
- Aminobenzoic acid or derivatives
- Benzoic acid or derivatives
- Benzoyl
- Benzenoid
- Dicarboxylic acid or derivatives
- Monocyclic benzene moiety
- Tertiary carboxylic acid amide
- Tertiary amine
- Guanidine
- Carboxylic acid ester
- Carboxamide group
- Carboximidamide
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Imine
- Carbonyl group
- Amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|