|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001671 |
|---|
|
Identification |
|---|
| Name: |
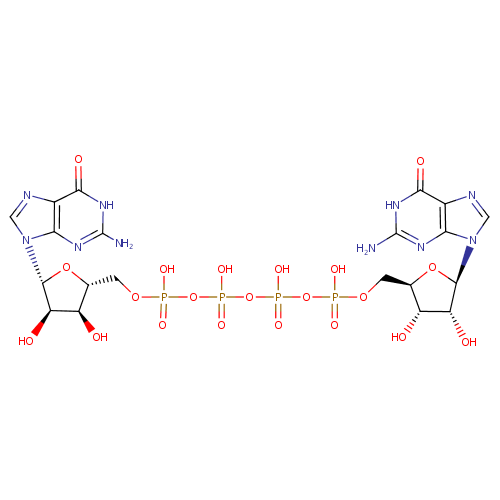
P1,P4-Bis(5'-guanosyl) tetraphosphate |
|---|
| Description: | Diguanosine tetraphosphate is a diguanosine polyphosphate. It consists of two guanosines joined by a chain of 4 phosphates. |
|---|
|
Structure |
|
|---|
| Synonyms: | - (ppG)2
- Bis(5'-guanosyl) tetraphosphate
- Bis(5'-guanosyl) tetraphosphoric acid
- Bis(guanylyl) diphosphate
- Bis(guanylyl) diphosphoric acid
- Diguanosine tetraphosphate
- Diguanosine tetraphosphoric acid
- G(5')P4(5')G
- GP4G
- GppppG
- Guanosine(5')tetraphospho(5')guanosine
- P1,P4-bis(5'-guanosyl) tetrahydrogen tetraphosphate
- P1,P4-Bis(5'-guanosyl) tetrahydrogen tetraphosphoric acid
- P1,P4-Bis(5'-guanosyl) tetraphosphate
- P1,P4-Bis(5'-guanosyl) tetraphosphoric acid
- [5-(2-amino-6-Hydroxy-purin-9-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-[[[[5-(2-amino-6-hydroxy-purin-9-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-hydroxy-phosphoryl]oxy-hydroxy-phosphoryl]oxy-hydroxy-phosphoryl]oxy-phosphinate
- [5-(2-amino-6-hydroxy-purin-9-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-[[[[5-(2-amino-6-hydroxy-purin-9-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-hydroxy-phosphoryl]oxy-hydroxy-phosphoryl]oxy-hydroxy-phosphoryl]oxy-phosphinic acid
|
|---|
|
Chemical Formula: |
C20H28N10O21P4 |
|---|
| Average Molecular Weight: |
868.3858 |
|---|
| Monoisotopic Molecular
Weight: |
868.038094056 |
|---|
| InChI Key: |
OLGWXCQXRSSQPO-MHARETSRSA-N |
|---|
| InChI: | InChI=1S/C20H28N10O21P4/c21-19-25-13-7(15(35)27-19)23-3-29(13)17-11(33)9(31)5(47-17)1-45-52(37,38)49-54(41,42)51-55(43,44)50-53(39,40)46-2-6-10(32)12(34)18(48-6)30-4-24-8-14(30)26-20(22)28-16(8)36/h3-6,9-12,17-18,31-34H,1-2H2,(H,37,38)(H,39,40)(H,41,42)(H,43,44)(H3,21,25,27,35)(H3,22,26,28,36)/t5-,6-,9-,10-,11-,12-,17-,18-/m1/s1 |
|---|
| CAS
number: |
4130-19-2 |
|---|
| IUPAC Name: | {[(2R,3S,4R,5R)-5-(2-amino-6-oxo-6,9-dihydro-1H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}({[({[({[(2R,3S,4R,5R)-5-(2-amino-6-oxo-6,9-dihydro-1H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)(hydroxy)phosphoryl]oxy})phosphinic acid |
|---|
|
Traditional IUPAC Name: |
diguanosine tetraphosphate |
|---|
| SMILES: | NC1=NC2=C(N=CN2[C@@H]2O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(=O)OP(O)(=O)OC[C@H]3O[C@H]([C@H](O)[C@@H]3O)N3C=NC4=C3N=C(N)NC4=O)[C@@H](O)[C@H]2O)C(=O)N1 |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as (5'->5')-dinucleotides. These are dinucleotides where the two bases are connected via a (5'->5')-phosphodiester linkage. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
|
Class |
(5'->5')-dinucleotides |
|---|
| Sub Class | Not Available |
|---|
|
Direct Parent |
(5'->5')-dinucleotides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- (5'->5')-dinucleotide
- Purine ribonucleoside polyphosphate
- Purine nucleotide sugar
- N-glycosyl compound
- Glycosyl compound
- Organic pyrophosphate
- Monosaccharide phosphate
- Hypoxanthine
- 6-oxopurine
- Purine
- Imidazopyrimidine
- Monoalkyl phosphate
- Pyrimidone
- Alkyl phosphate
- Pyrimidine
- Primary aromatic amine
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic phosphate
- N-substituted imidazole
- Monosaccharide
- Saccharide
- Heteroaromatic compound
- Vinylogous amide
- Oxolane
- Imidazole
- Azole
- Secondary alcohol
- Lactam
- 1,2-diol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Amine
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Jankowski J, Hagemann J, Tepel M, van Der Giet M, Stephan N, Henning L, Gouni-Berthold I, Sachinidis A, Zidek W, Schluter H: Dinucleotides as growth-promoting extracellular mediators. Presence of dinucleoside diphosphates Ap2A, Ap2G, and Gp2G in releasable granules of platelets. J Biol Chem. 2001 Mar 23;276(12):8904-9. Epub 2000 Dec 13. Pubmed: 11115507
- Ralevic V, Jankowski J, Schluter H: Structure-activity relationships of diadenosine polyphosphates (Ap(n)As), adenosine polyphospho guanosines (Ap(n)Gs) and guanosine polyphospho guanosines (Gp(n)Gs) at P2 receptors in the rat mesenteric arterial bed. Br J Pharmacol. 2001 Nov;134(5):1073-83. Pubmed: 11682456
- van der Giet M, Westhoff T, Cinkilic O, Jankowski J, Schluter H, Zidek W, Tepel M: The critical role of adenosine and guanosine in the affinity of dinucleoside polyphosphates to P(2X)-receptors in the isolated perfused rat kidney. Br J Pharmacol. 2001 Jan;132(2):467-74. Pubmed: 11159696
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|