|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001663 |
|---|
|
Identification |
|---|
| Name: |
Nitrous oxide |
|---|
| Description: | Nitrous oxide, commonly known as laughing gas, is a chemical compound with the formula N2O. It is an oxide of nitrogen. At room temperature, it is a colorless, non-flammable gas, with a slightly sweet odor and taste. N2O is produced naturally by microorganisms in soil through various types of nitrification and denitrification processes. (Wikipedia) Nitrous oxide production by Pseudomonas aeruginosa seems to result from the reduction of nitrite (NO2-) by nitrate (NO3-) reductase. (PMID 6347062) |
|---|
|
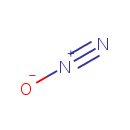
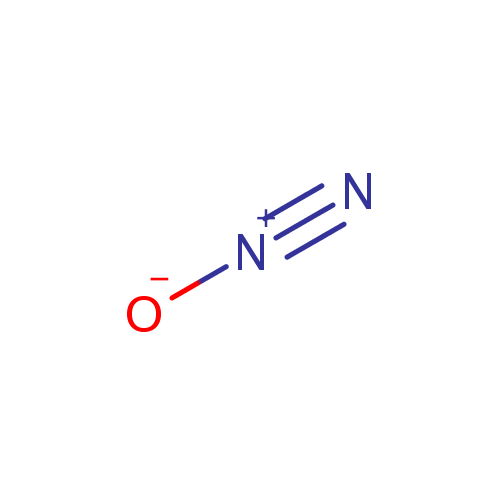
Structure |
|
|---|
| Synonyms: | - Diazyne 1-oxide
- Dinitrogen monoxide
- Dinitrogen oxide
- Distickstoffmonoxid
- Factitious air
- Gaz hilarant
- Hyponitrous acid anhydride
- Lachgas
- Laughing gas
- N2O
- Nitral
- Nitrogen monoxide
- Nitrogen oxide
- Nitrogen oxide (N2O)
- Nitrogen protoxide
- Nitrogenium oxydulatum
- Nitrous oxide
- NNO
- Oxidodinitrogen(NN)
- Oxyde nitreux
- Protoxyde D'azote
- Stickstoff(1)-oxid
|
|---|
|
Chemical Formula: |
N2O |
|---|
| Average Molecular Weight: |
44.0128 |
|---|
| Monoisotopic Molecular
Weight: |
44.001062632 |
|---|
| InChI Key: |
GQPLMRYTRLFLPF-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/N2O/c1-2-3 |
|---|
| CAS
number: |
10024-97-2 |
|---|
| IUPAC Name: | diazooxidane |
|---|
|
Traditional IUPAC Name: |
nitrous oxide |
|---|
| SMILES: | [O-][N+]#N |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of inorganic compounds known as homogeneous other non-metal compounds. These are inorganic non-metallic compounds in which the largest atom belongs to the class of 'other nonmetals'. |
|---|
|
Kingdom |
Inorganic compounds |
|---|
| Super Class | Homogeneous non-metal compounds |
|---|
|
Class |
Homogeneous other non-metal compounds |
|---|
| Sub Class | Not Available |
|---|
|
Direct Parent |
Homogeneous other non-metal compounds |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Homogeneous other non metal
- Inorganic oxide
- Acyclic compound
|
|---|
| Molecular Framework |
Acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Liquid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
-90.81 °C |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Smith, M. S. (1983). "Nitrous oxide production by Escherichia coli is correlated with nitrate reductase activity." Appl Environ Microbiol 45:1545-1547. Pubmed: 6347062
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|