|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001641 |
|---|
|
Identification |
|---|
| Name: |
Isethionic acid |
|---|
| Description: | Isethionic acid (C2H6O4S) is a short chain alkane sulfonate containing hydroxy group, is a water soluble liquid used in the manufacture of mild, biodegradable and high foaming anionic surfactants which provides gentle cleansing and soft skin feel.; A colorless, syrupy, strongly acidic liquid that can form detergents with oleic acid. The Pseudomonas aeruginosa ssuEADCB gene cluster is required for the utilization of alkanesulfonates (such as isethionic acid) as sulfur sources, and is expressed under conditions of sulfate or cysteine starvation. |
|---|
|
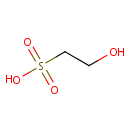
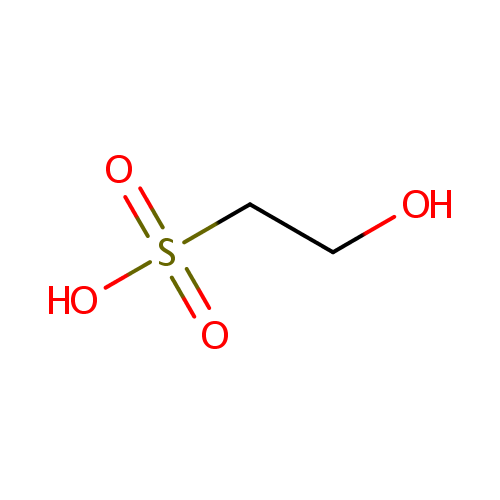
Structure |
|
|---|
| Synonyms: | - (2-Hydroxyethyl)sulfonate
- (2-Hydroxyethyl)sulfonic acid
- (2-Hydroxyethyl)sulphonate
- (2-Hydroxyethyl)sulphonic acid
- 2-Hydroxy-Ethanesulfonate
- 2-Hydroxy-Ethanesulfonic acid
- 2-Hydroxy-Ethanesulphonate
- 2-Hydroxy-Ethanesulphonic acid
- 2-Hydroxyethane-1-sulfonate
- 2-Hydroxyethane-1-sulfonic acid
- 2-Hydroxyethane-1-sulphonate
- 2-Hydroxyethane-1-sulphonic acid
- 2-Hydroxyethanesulfonate
- 2-Hydroxyethanesulfonic acid
- 2-Hydroxyethanesulphonate
- 2-Hydroxyethanesulphonic acid
- 2-Hydroxyethylsulfonate
- 2-Hydroxyethylsulfonic acid
- 2-Hydroxyethylsulphonate
- 2-Hydroxyethylsulphonic acid
- b-Hydroxyethanesulfonate
- b-Hydroxyethanesulfonic acid
- b-Hydroxyethanesulphonate
- b-Hydroxyethanesulphonic acid
- Beta-Hydroxyethanesulfonate
- Beta-Hydroxyethanesulfonic acid
- Beta-Hydroxyethanesulphonate
- Beta-Hydroxyethanesulphonic acid
- Ethanolsulfonate
- Ethanolsulfonic acid
- Ethanolsulphonate
- Ethanolsulphonic acid
- Hydroxyethylsulfonate
- Hydroxyethylsulfonic acid
- Hydroxyethylsulphonate
- Hydroxyethylsulphonic acid
- Isethionate
- Isethionate sodium salt
- Isethionic acid
- Isethionic acid sodium salt
- Kyselina isethionova
- Potassium 2-hydroxyethanesulfonate
- Potassium 2-hydroxyethanesulfonic acid
- Potassium 2-hydroxyethanesulphonate
- Potassium 2-hydroxyethanesulphonic acid
- Potassium isethionate
- Potassium isethionic acid
- Sodium 2-hydroxyethanesulfonate
- Sodium 2-hydroxyethanesulfonic acid
- Sodium 2-hydroxyethanesulphonate
- Sodium 2-hydroxyethanesulphonic acid
- Sodium 2-hydroxyethyl sulfonate
- Sodium 2-hydroxyethyl sulfonic acid
- Sodium 2-hydroxyethyl sulphonate
- Sodium 2-hydroxyethyl sulphonic acid
- Sodium b-hydroxyethanesulfonate
- Sodium b-hydroxyethanesulfonic acid
- Sodium b-hydroxyethanesulphonate
- Sodium b-hydroxyethanesulphonic acid
- Sodium beta-hydroxyethanesulfonate
- Sodium beta-hydroxyethanesulfonic acid
- Sodium beta-hydroxyethanesulphonate
- Sodium beta-hydroxyethanesulphonic acid
- Sodium isethionate
- Sodium isethionic acid
- Sodium β-hydroxyethanesulfonate
- Sodium β-hydroxyethanesulfonic acid
- Sodium β-hydroxyethanesulphonate
- Sodium β-hydroxyethanesulphonic acid
- β-Hydroxyethanesulfonate
- β-Hydroxyethanesulfonic acid
- β-Hydroxyethanesulphonate
- β-Hydroxyethanesulphonic acid
|
|---|
|
Chemical Formula: |
C2H6O4S |
|---|
| Average Molecular Weight: |
126.132 |
|---|
| Monoisotopic Molecular
Weight: |
125.99867937 |
|---|
| InChI Key: |
SUMDYPCJJOFFON-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C2H6O4S/c3-1-2-7(4,5)6/h3H,1-2H2,(H,4,5,6) |
|---|
| CAS
number: |
107-36-8 |
|---|
| IUPAC Name: | 2-hydroxyethane-1-sulfonic acid |
|---|
|
Traditional IUPAC Name: |
sodium isethionate |
|---|
| SMILES: | OCCS(O)(=O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as sulfonic acids. These are compounds containing the sulfonic acid group, which has the general structure RS(=O)2OH (R is not a hydrogen atom). |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Sulfonic acids and derivatives |
|---|
| Sub Class | Sulfonic acids |
|---|
|
Direct Parent |
Sulfonic acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alkanesulfonic acid
- Sulfonyl
- Sulfonic acid
- Hydrocarbon derivative
- Primary alcohol
- Organosulfur compound
- Organooxygen compound
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
< 25 °C |
|---|
| Experimental Properties: |
| Property | Value | Source |
|---|
| Water Solubility: | 1000 mg/mL | PhysProp |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kersten A, Althaus C, Seitz HM, Pfahl HG, Sundmacher R: [Bilateral microsporidial keratitis in an HIV-positive patient with AIDS stage infection] Klin Monatsbl Augenheilkd. 1998 Jun;212(6):476-9. Pubmed: 9715470
|
|---|
| Synthesis Reference: |
Chen, Zhiwen; Liao, Xiangxu; Qian, Weiqiang; et al. Process for preparation of hydroxyalkanesulfonic acids for plating solutions. Faming Zhuanli Shenqing Gongkai Shuomingshu (1990), 6 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|