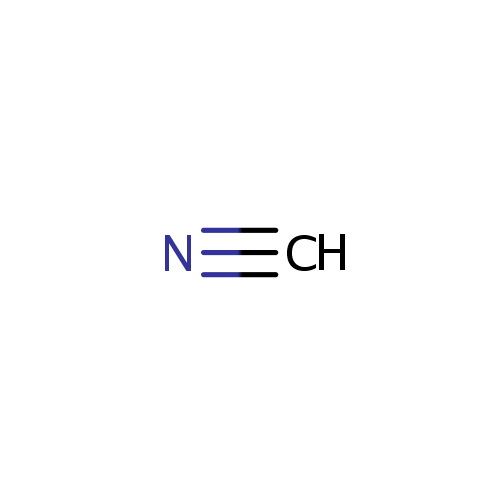Hydrogen cyanide (PAMDB001639)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB001639 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Hydrogen cyanide | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | HCN is formed in interstellar clouds through one of two major pathways: via a neutral-neutral reaction (CH2 + N <=> HCN + H) and via dissociative recombination (HCNH+ + e- <=> HCN + H). The dissociative recombination pathway is dominant by 30%; however, the HCNH+ must be in its linear form. Dissociative recombination with its structural isomer, H2NC+ produces hydrogen isocyanide (HNC), exclusively.; HCN is produced on an industrial scale and is a highly valuable precursor to many chemical compounds ranging from polymers to pharmaceuticals.; Hydrogen cyanide (with the historical common name of Prussic acid) is an inorganic compound with chemical formula HCN. It is a colorless, extremely poisonous liquid that boils slightly above room temperature at 26 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | CHN | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 27.0253 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 27.010899037 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | LELOWRISYMNNSU-UHFFFAOYSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/CHN/c1-2/h1H | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 74-90-8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | formonitrile | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | hydrogen cyanide | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | C#N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as nitriles. These are compounds having the structure RC#N; thus C-substituted derivatives of hydrocyanic acid, HC#N. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organonitrogen compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Nitriles | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Nitriles | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Liquid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | -13.4 °C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Hydrogen cyanide + Thiosulfate > Hydrogen ion + Sulfite + Thiocyanate Hydrogen cyanide + 3-Mercaptopyruvic acid + Cyanide <> Hydrogen ion + Pyruvic acid + Thiocyanate Hydrogen cyanide + 3-Mercaptopyruvic acid <> Thiocyanate + Pyruvic acid Hydrogen cyanide + 3-Mercaptopyruvic acid Hydrogen ion + Pyruvic acid + Thiocyanate <i>S</i>-sulfanyl-[acceptor] + Hydrogen cyanide an unsulfurated sulfur acceptor + Thiocyanate + Hydrogen ion Thiosulfate + Hydrogen cyanide > Sulfite + Thiocyanate | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in thiosulfate sulfurtransferase activity
- Specific function:
- Catalyzes, although with low efficiency, the sulfur transfer reaction from thiosulfate to cyanide. The relatively low affinity of glpE for both thiosulfate and cyanide suggests that these compounds are not the physiological substrates. Thioredoxin 1 or related dithiol proteins could instead be the physiological sulfur-acceptor substrate. Possible association with the metabolism of glycerol-phosphate remains to be elucidated
- Gene Name:
- glpE
- Locus Tag:
- PA0589
- Molecular weight:
- 12 kDa
Reactions
| Thiosulfate + cyanide = sulfite + thiocyanate. |