|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001608 |
|---|
|
Identification |
|---|
| Name: |
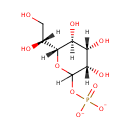
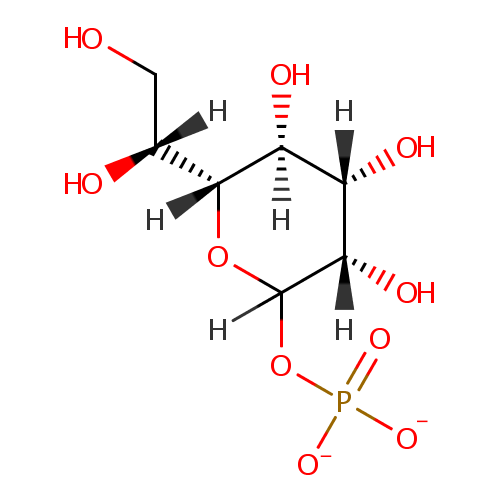
D-Glycero-D-manno-heptose 1-phosphate |
|---|
| Description: | D-glycero-D-manno-heptose 1-phosphate is a member of the chemical class known as Hexoses. These are monosaccharides in which the sugar unit is a hexose. D-Glycero-D-manno-heptose 1-phosphate is involved in the biosynthesis of the lipopolysaccharide core precursor ADP-L-glycero-D-manno-heptose. (PMID 10629197) |
|---|
|
Structure |
|
|---|
| Synonyms: | - 1-O-Phosphono-D-glycero-Dmanno-heptopyranose
- D-glycero-b-D-manno-Heptose 1-phosphate
- D-glycero-b-D-manno-Heptose 1-phosphoric acid
- D-Glycero-beta-D-manno-Heptose 1-phosphate
- D-glycero-beta-D-manno-Heptose 1-phosphoric acid
- D-Glycero-D-manno-Heptose 1-phosphate
- D-glycero-D-manno-Heptose 1-phosphoric acid
- D-Glycero-Dmanno-heptopyranose 1-(dihydrogen phosphate)
- D-glycero-dmanno-Heptopyranose 1-(dihydrogen phosphoric acid)
- D-glycero-β-D-manno-Heptose 1-phosphate
- D-glycero-β-D-manno-Heptose 1-phosphoric acid
|
|---|
|
Chemical Formula: |
C7H13O10P |
|---|
| Average Molecular Weight: |
288.1459 |
|---|
| Monoisotopic Molecular
Weight: |
288.024633148 |
|---|
| InChI Key: |
KMEJCSKJXSBBAN-NNPWBXLPSA-L |
|---|
| InChI: | InChI=1S/C7H15O10P/c8-1-2(9)6-4(11)3(10)5(12)7(16-6)17-18(13,14)15/h2-12H,1H2,(H2,13,14,15)/p-2/t2-,3+,4+,5+,6-,7?/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (3S,4S,5S,6R)-6-[(1R)-1,2-dihydroxyethyl]-3,4,5-trihydroxyoxan-2-yl phosphate |
|---|
|
Traditional IUPAC Name: |
(3S,4S,5S,6R)-6-[(1R)-1,2-dihydroxyethyl]-3,4,5-trihydroxyoxan-2-yl phosphate |
|---|
| SMILES: | [H][C@@](O)(CO)[C@@]1([H])OC([H])(OP([O-])([O-])=O)[C@@]([H])(O)[C@@]([H])(O)[C@]1([H])O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as monosaccharide phosphates. These are monosaccharides comprising a phosphated group linked to the carbohydrate unit. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organooxygen compounds |
|---|
|
Class |
Carbohydrates and carbohydrate conjugates |
|---|
| Sub Class | Monosaccharides |
|---|
|
Direct Parent |
Monosaccharide phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Monosaccharide phosphate
- Alkyl phosphate
- Phosphoric acid ester
- Oxane
- Organic phosphoric acid derivative
- Organic phosphate
- Secondary alcohol
- Polyol
- 1,2-diol
- Oxacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Primary alcohol
- Alcohol
- Organic anion
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | 28137 | | HMDB ID | Not Available | | Pubchem Compound ID | 46878581 | | Kegg ID | C07838 | | ChemSpider ID | 26331785 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|