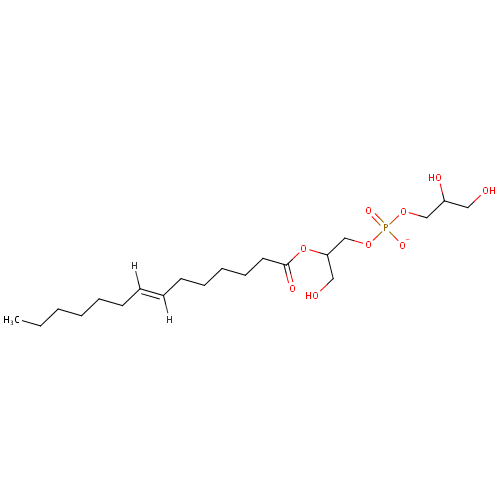
2-Acyl-sn-glycero-3-phosphoglycerol (N-C14:1) (PAMDB001557)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB001557 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 2-Acyl-sn-glycero-3-phosphoglycerol (N-C14:1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | 2-Acyl-sn-glycero-3-phosphoglycerol (n-C14:1) belongs to the class of Lysophosphatidylglycerols. These are glycerophosphoglycerols (molecules containing a glycerol moiety attached to the phosphate group linked to a glycerol) in which only one fatty acid is bonded to the 1-glycerol moiety (through an ester linkage). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C20H38O9P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 453.4841 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 453.225344326 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | BHICCPXVDIQMCF-BQYQJAHWSA-M | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C20H39O9P/c1-2-3-4-5-6-7-8-9-10-11-12-13-20(24)29-19(15-22)17-28-30(25,26)27-16-18(23)14-21/h7-8,18-19,21-23H,2-6,9-17H2,1H3,(H,25,26)/p-1/b8-7+ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 1-[(2,3-dihydroxypropyl phosphonato)oxy]-3-hydroxypropan-2-yl (7E)-tetradec-7-enoate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | 1-[(2,3-dihydroxypropyl phosphonato)oxy]-3-hydroxypropan-2-yl (7E)-tetradec-7-enoate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | [H]\C(CCCCCC)=C(\[H])CCCCCC(=O)OC(CO)COP([O-])(=O)OCC(O)CO | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as lysophosphatidylglycerols. These are glycerophosphoglycerols (molecules containing a glycerol moiety attached to the phosphate group linked to a glycerol) in which only one fatty acid is bonded to the 1-glycerol moiety (through an ester linkage). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Lipids and lipid-like molecules | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Glycerophospholipids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Glycerophosphoglycerols | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Lysophosphatidylglycerols | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 2-Acyl-sn-glycero-3-phosphoglycerol (N-C14:1) + Adenosine triphosphate + Tetradecenoate (N-C14:1) > Adenosine monophosphate + PG(14:1(7Z)/14:1(7Z)) + Pyrophosphate Water + PG(14:1(7Z)/14:1(7Z)) > 2-Acyl-sn-glycero-3-phosphoglycerol (N-C14:1) + Hydrogen ion + Tetradecenoate (N-C14:1) 2-Acyl-sn-glycero-3-phosphoglycerol (N-C14:1) + Water > Glycerophosphoglycerol + Hydrogen ion + Tetradecenoate (N-C14:1) 2-Acyl-sn-glycero-3-phosphoglycerol (N-C14:1) + PG(14:1(7Z)/14:1(7Z)) > Acyl phosphatidylglycerol (N-C14:1) + Glycerophosphoglycerol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Lipid transport and metabolism
- Specific function:
- 2-lysophosphatidylcholine + H(2)O = glycerophosphocholine + a carboxylate
- Gene Name:
- pldB
- Locus Tag:
- PA5089
- Molecular weight:
- 83.4 kDa
Reactions
| 2-lysophosphatidylcholine + H(2)O = glycerophosphocholine + a carboxylate. |