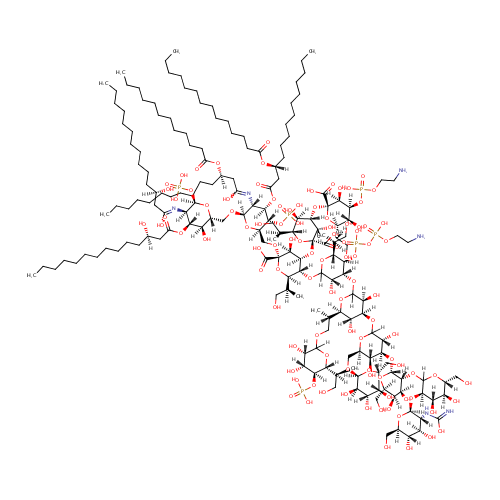
|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001506 |
|---|
|
Identification |
|---|
| Name: |
Ra-LPS |
|---|
| Description: | Lipopolysaccharides (LPS), also known as lipoglycans, are large molecules consisting of a lipid and a polysaccharide joined by a covalent bond; they are found in the outer membrane of Gram-negative bacteria, act as endotoxins and elicit strong immune responses in animals. There are 2 forms of LPS: smooth-form and rough-form. The smooth-form (S-form) LPS consists of three parts: O antigen (or O polysaccharide), core region and lipid A. The rough-form (R-form) LPS only consists of core region and lipid A. The Core domain always contains an oligosaccharide component which attaches directly to lipid A and commonly contains sugars such as heptose and 3-deoxy-D-mannooctulosonic acid (also known as KDO, keto-deoxyoctulosonate). Depending on the quantity and composition of the sugars in the core region, Pseudomonas aeruginosa K-12 R-form LPS can be classified as Ra, Rb1, Rb2, Rc, Rd1, Rd2, and Re-LPS. LPS is first synthesized as KDO-lipid A or Re-LPS. Adding more sugars to the core region gives rise to Rd, Rc, Rb and Ra-LPS. (Wikipedia; PMID: 17403049; PMID: 17364071; PMDI: 20203010) |
|---|
|
Structure |
|
|---|
| Synonyms: | - Core oligosaccharide
- Core oligosaccharide lipid A
- Endotoxin Lipopolysaccharide core
- Endotoxin LPS core
- Lipopolysaccharide
- Lipopolysaccharide core endotoxin
- Lipopolysaccharide Ra component
- LPS core
|
|---|
|
Chemical Formula: |
C180H328N6O98P6 |
|---|
| Average Molecular Weight: |
4330.3543 |
|---|
| Monoisotopic Molecular
Weight: |
4327.929256554 |
|---|
| InChI Key: |
CQMSKDRUAIEDAN-FCHZNYOBSA-N |
|---|
| InChI: | InChI=1S/C180H328N6O98P6/c1-13-19-25-31-37-43-44-50-56-62-68-74-116(201)255-105(72-66-60-54-48-41-35-29-23-17-5)82-118(203)264-149-121(185-114(199)81-104(71-65-59-53-47-40-34-28-22-16-4)254-115(200)73-67-61-55-49-42-36-30-24-18-6)164(249-93-110-126(208)148(263-117(202)80-103(197)70-64-58-52-46-39-33-27-21-15-3)120(166(260-110)283-287(239,240)241)184-113(198)79-102(196)69-63-57-51-45-38-32-26-20-14-2)262-112(147(149)279-285(233,234)235)95-251-178(174(226)227)163(225)160(278-179(175(228)229)161(223)154(136(218)142(274-179)96(7)83-187)277-180(176(230)231)162(224)155(281-288(242,243)252-77-75-181)137(219)143(275-180)97(8)84-188)158(146(276-178)100(11)87-191)273-171-140(222)152(159(145(267-171)99(10)86-190)282-290(246,247)284-289(244,245)253-78-76-182)270-170-139(221)151(135(217)141(265-170)101(12)92-248-168-134(216)132(214)153(280-286(236,237)238)144(266-168)98(9)85-189)268-169-138(220)150(127(209)111(261-169)94-250-167-133(215)129(211)123(205)107(89-193)257-167)269-172-157(131(213)125(207)108(90-194)258-172)272-173-156(130(212)124(206)109(91-195)259-173)271-165-119(186-177(183)232)128(210)122(204)106(88-192)256-165/h96-112,119-173,187-197,204-225H,13-95,181-182H2,1-12H3,(H,184,198)(H,185,199)(H,226,227)(H,228,229)(H,230,231)(H,242,243)(H,244,245)(H,246,247)(H3,183,186,232)(H2,233,234,235)(H2,236,237,238)(H2,239,240,241)/t96-,97-,98-,99-,100-,101-,102+,103+,104+,105+,106+,107+,108+,109+,110+,111+,112+,119+,120+,121+,122+,123-,124+,125-,126+,127+,128+,129-,130-,131-,132+,133+,134-,135+,136+,137+,138+,139-,140-,141+,142+,143+,144+,145+,146+,147+,148+,149+,150-,151-,152+,153-,154-,155-,156+,157+,158+,159+,160-,161-,162-,163-,164+,165-,166+,167-,168?,169?,170?,171?,172+,173?,178+,179-,180-/m0/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (2R,3S,4R,5R,6R)-5-{[(3S,4R,5R,6R)-5-[({[(2-aminoethoxy)(hydroxy)phosphoryl]oxy}(hydroxy)phosphoryl)oxy]-4-{[(3S,4S,5R,6R)-4-{[(3R,4S,5R,6R)-4-{[(2R,3R,4S,5R,6R)-3-{[(3R,4S,5S,6R)-3-{[(2S,3R,4R,5S,6R)-4,5-dihydroxy-3-[(C-hydroxycarbonimidoyl)amino]-6-(hydroxymethyl)oxan-2-yl]oxy}-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,5-dihydroxy-6-({[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}methyl)oxan-2-yl]oxy}-6-[(2S)-1-{[(3S,4R,5S,6R)-3,4-dihydroxy-6-[(2S)-1-hydroxypropan-2-yl]-5-(phosphonooxy)oxan-2-yl]oxy}propan-2-yl]-3,5-dihydroxyoxan-2-yl]oxy}-3-hydroxy-6-[(2S)-1-hydroxypropan-2-yl]oxan-2-yl]oxy}-4-{[(2S,3S,4S,5R,6R)-4-{[(2S,3S,4S,5R,6R)-4-{[(2-aminoethoxy)(hydroxy)phosphoryl]oxy}-2-carboxy-3,5-dihydroxy-6-[(2S)-1-hydroxypropan-2-yl]oxan-2-yl]oxy}-2-carboxy-3,5-dihydroxy-6-[(2S)-1-hydroxypropan-2-yl]oxan-2-yl]oxy}-2-{[(2R,3S,4R,5R,6R)-6-{[(2R,3S,4R,5R,6R)-5-{[(3R)-1,3-dihydroxytetradecylidene]amino}-3-hydroxy-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-6-(phosphonooxy)oxan-2-yl]methoxy}-5-{[(3R)-3-(dodecanoyloxy)-1-hydroxytetradecylidene]amino}-3-(phosphonooxy)-4-{[(3R)-3-(tetradecanoyloxy)tetradecanoyl]oxy}oxan-2-yl]methoxy}-3-hydroxy-6-[(2S)-1-hydroxypropan-2-yl]oxane-2-carboxylic acid |
|---|
|
Traditional IUPAC Name: |
(2R,3S,4R,5R,6R)-5-{[(3S,4R,5R,6R)-5-({[2-aminoethoxy(hydroxy)phosphoryl]oxy(hydroxy)phosphoryl}oxy)-4-{[(3S,4S,5R,6R)-4-{[(3R,4S,5R,6R)-4-{[(2R,3R,4S,5R,6R)-3-{[(3R,4S,5S,6R)-3-{[(2S,3R,4R,5S,6R)-4,5-dihydroxy-3-(C-hydroxycarbonimidoylamino)-6-(hydroxymethyl)oxan-2-yl]oxy}-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,5-dihydroxy-6-({[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}methyl)oxan-2-yl]oxy}-6-[(2S)-1-{[(3S,4R,5S,6R)-3,4-dihydroxy-6-[(2S)-1-hydroxypropan-2-yl]-5-(phosphonooxy)oxan-2-yl]oxy}propan-2-yl]-3,5-dihydroxyoxan-2-yl]oxy}-3-hydroxy-6-[(2S)-1-hydroxypropan-2-yl]oxan-2-yl]oxy}-4-{[(2S,3S,4S,5R,6R)-4-{[(2S,3S,4S,5R,6R)-4-{[2-aminoethoxy(hydroxy)phosphoryl]oxy}-2-carboxy-3,5-dihydroxy-6-[(2S)-1-hydroxypropan-2-yl]oxan-2-yl]oxy}-2-carboxy-3,5-dihydroxy-6-[(2S)-1-hydroxypropan-2-yl]oxan-2-yl]oxy}-2-{[(2R,3S,4R,5R,6R)-6-{[(2R,3S,4R,5R,6R)-5-{[(3R)-1,3-dihydroxytetradecylidene]amino}-3-hydroxy-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-6-(phosphonooxy)oxan-2-yl]methoxy}-5-{[(3R)-3-(dodecanoyloxy)-1-hydroxytetradecylidene]amino}-3-(phosphonooxy)-4-{[(3R)-3-(tetradecanoyloxy)tetradecanoyl]oxy}oxan-2-yl]methoxy}-3-hydroxy-6-[(2S)-1-hydroxypropan-2-yl]oxane-2-carboxylic acid |
|---|
| SMILES: | [H][C@@](O)(CCCCCCCCCCC)CC(=O)O[C@@]1([H])[C@]([H])(O)[C@@]([H])(CO[C@]2([H])O[C@]([H])(CO[C@]3(O[C@]([H])([C@@]([H])(C)CO)[C@@]([H])(OC4([H])O[C@]([H])([C@@]([H])(C)CO)[C@@]([H])(OP(O)(=O)OP(O)(=O)OCCN)[C@]([H])(OC5([H])O[C@]([H])([C@@]([H])(C)COC6([H])O[C@]([H])([C@@]([H])(C)CO)[C@@]([H])(OP(O)(O)=O)[C@]([H])(O)[C@]6([H])O)[C@@]([H])(O)[C@]([H])(OC6([H])O[C@]([H])(CO[C@@]7([H])O[C@]([H])(CO)[C@]([H])(O)[C@]([H])(O)[C@@]7([H])O)[C@@]([H])(O)[C@]([H])(O[C@@]7([H])O[C@]([H])(CO)[C@]([H])(O)[C@]([H])(O)[C@@]7([H])OC7([H])O[C@]([H])(CO)[C@@]([H])(O)[C@]([H])(O)[C@@]7([H])O[C@]7([H])O[C@]([H])(CO)[C@@]([H])(O)[C@]([H])(O)[C@@]7([H])NC(O)=N)[C@@]6([H])O)[C@]5([H])O)[C@]4([H])O)[C@]([H])(O[C@]4(O[C@]([H])([C@@]([H])(C)CO)[C@@]([H])(O)[C@]([H])(O[C@]5(O[C@]([H])([C@@]([H])(C)CO)[C@@]([H])(O)[C@]([H])(OP(O)(=O)OCCN)[C@]5([H])O)C(O)=O)[C@]4([H])O)C(O)=O)[C@]3([H])O)C(O)=O)[C@@]([H])(OP(O)(O)=O)[C@]([H])(OC(=O)C[C@@]([H])(CCCCCCCCCCC)OC(=O)CCCCCCCCCCCCC)[C@@]2([H])N=C(O)C[C@@]([H])(CCCCCCCCCCC)OC(=O)CCCCCCCCCCC)O[C@]([H])(OP(O)(O)=O)[C@]1([H])N=C(O)C[C@]([H])(O)CCCCCCCCCCC |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as polysaccharides. These are compounds containing more than ten saccharide units. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic Polymers |
|---|
|
Class |
Polysaccharides |
|---|
| Sub Class | Not Available |
|---|
|
Direct Parent |
Polysaccharides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Polysaccharide
- Saccharolipid
- Hexose phosphate
- N-acyl-alpha-hexosamine
- Fatty acyl glycoside
- C-glucuronide
- Glucuronic acid or derivatives
- Alkyl glycoside
- O-glycosyl compound
- C-glycosyl compound
- Glycosyl compound
- Organic pyrophosphate
- Pyran carboxylic acid
- Pyran carboxylic acid or derivatives
- Phosphoethanolamine
- Monoalkyl phosphate
- Beta-hydroxy acid
- Ketal
- Dialkyl phosphate
- Fatty acid ester
- Alkyl phosphate
- Organic phosphate
- Organic phosphoric acid derivative
- Pyran
- Hydroxy acid
- Phosphoric acid ester
- Fatty acyl
- 1,2-diol
- Amino acid or derivatives
- Amino acid
- Carboxylic acid ester
- Secondary alcohol
- Isourea
- Polyol
- Acetal
- Oxacycle
- Carboximidic acid derivative
- Carboxylic acid derivative
- Carboxylic acid
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboximidamide
- Primary alcohol
- Carbonyl group
- Primary aliphatic amine
- Amine
- Organooxygen compound
- Alcohol
- Hydrocarbon derivative
- Organonitrogen compound
- Organic oxide
- Primary amine
- Imine
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -8 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Membrane |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Holst, O. (2007). "The structures of core regions from enterobacterial lipopolysaccharides - an update." FEMS Microbiol Lett 271:3-11. Pubmed: 17403049
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Zubova, S. V., Prokhorenko, I. R. (2006). "Use of colorimetric method for evaluation of LPS of different structure." Bull Exp Biol Med 141:765-767. Pubmed: 17364071
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|