|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001395 |
|---|
|
Identification |
|---|
| Name: |
NMNH |
|---|
| Description: | NMNH is a member of the chemical class known as Nicotinamide Nucleotides. These are pyridine nucleotides, in which the pyridine base is nicotinamide or a derivative thereof. |
|---|
|
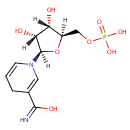
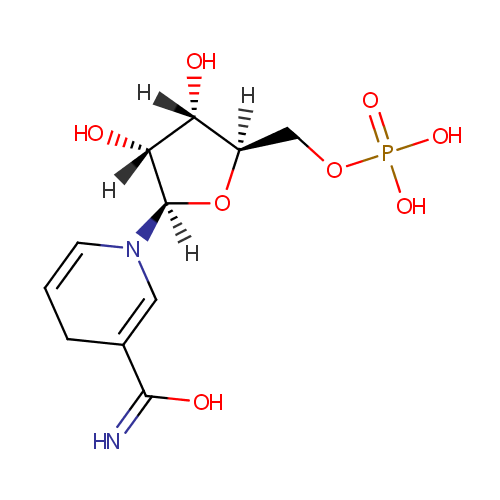
Structure |
|
|---|
| Synonyms: | - Nicotinamide mononucleotide (reduced)
|
|---|
|
Chemical Formula: |
C11H17N2O8P |
|---|
| Average Molecular Weight: |
336.235 |
|---|
| Monoisotopic Molecular
Weight: |
336.072252042 |
|---|
| InChI Key: |
XQHMUSRSLNRVGA-TURQNECASA-N |
|---|
| InChI: | InChI=1S/C11H17N2O8P/c12-10(16)6-2-1-3-13(4-6)11-9(15)8(14)7(21-11)5-20-22(17,18)19/h1,3-4,7-9,11,14-15H,2,5H2,(H2,12,16)(H2,17,18,19)/t7-,8-,9-,11-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-[(phosphonooxy)methyl]oxolan-2-yl]-1,4-dihydropyridine-3-carboximidic acid |
|---|
|
Traditional IUPAC Name: |
1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-[(phosphonooxy)methyl]oxolan-2-yl]-4H-pyridine-3-carboximidic acid |
|---|
| SMILES: | [H][C@]1(COP(O)(O)=O)O[C@@]([H])(N2C=CCC(=C2)C(O)=N)[C@]([H])(O)[C@]1([H])O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as nicotinamide nucleotides. These are pyridine nucleotides, in which the pyridine base is nicotinamide or a derivative thereof. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
|
Class |
Pyridine nucleotides |
|---|
| Sub Class | Nicotinamide nucleotides |
|---|
|
Direct Parent |
Nicotinamide nucleotides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Nicotinamide-nucleotide
- N-glycosyl compound
- Glycosyl compound
- N-substituted nicotinamide
- Monosaccharide phosphate
- Dihydropyridinecarboxylic acid derivative
- Monoalkyl phosphate
- Dihydropyridine
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic phosphate
- Monosaccharide
- Hydropyridine
- Saccharide
- Oxolane
- Tertiary amine
- Secondary alcohol
- 1,2-diol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Enamine
- Carboximidic acid derivative
- Carboximidic acid
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Amine
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|