|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001379 |
|---|
|
Identification |
|---|
| Name: |
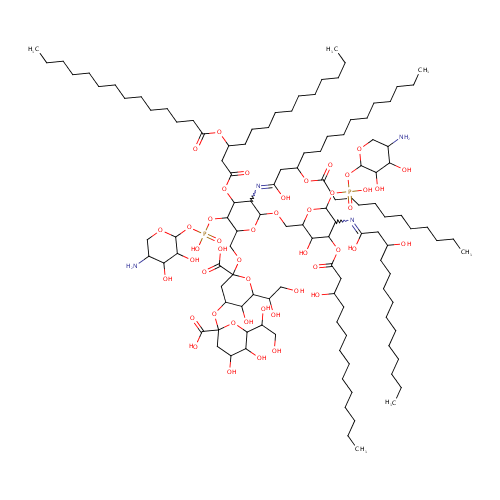
L-Ara4N-modified KDO2-Lipid A |
|---|
| Description: | L-ara4n-modified kdo2-lipid a is a member of the chemical class known as Hexose Oligosaccharides. These are oligosaccharides in which the saccharide units are hexoses. |
|---|
|
Structure |
|
|---|
| Synonyms: | - α-Kdo-(2→4)-α-Kdo-(2→6)-lipid IIA
- α-kdo-(2→4)-α-kdo-(2→6)-lipid iia
- a-kdo-(2->4)-a-kdo-(2->6)-lipid a
- Alpha-KDO-(2->4)-alpha-KDO-(2->6)-lipid A
- L-Ara4n-modified (kdo)2-lipid a
- L-Ara4N-modified (KDO)2-Lipid A
- α-kdo-(2->4)-α-kdo-(2->6)-lipid a
|
|---|
|
Chemical Formula: |
C120H220N4O45P2 |
|---|
| Average Molecular Weight: |
2500.9781 |
|---|
| Monoisotopic Molecular
Weight: |
2499.452484074 |
|---|
| InChI Key: |
RYVJLJVPSMBXLB-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C120H220N4O45P2/c1-7-13-19-25-31-37-38-44-50-56-62-68-96(135)158-84(66-60-54-48-42-35-29-23-17-11-5)72-98(137)162-112-100(124-94(133)71-83(65-59-53-47-41-34-28-22-16-10-4)157-95(134)67-61-55-49-43-36-30-24-18-12-6)113(160-92(110(112)166-170(149,150)168-115-106(143)101(138)85(121)77-153-115)80-156-119(117(145)146)74-90(104(141)109(164-119)89(131)76-126)163-120(118(147)148)73-87(129)103(140)108(165-120)88(130)75-125)155-79-91-105(142)111(161-97(136)70-82(128)64-58-52-46-40-33-27-21-15-9-3)99(123-93(132)69-81(127)63-57-51-45-39-32-26-20-14-8-2)114(159-91)167-171(151,152)169-116-107(144)102(139)86(122)78-154-116/h81-92,99-116,125-131,138-144H,7-80,121-122H2,1-6H3,(H,123,132)(H,124,133)(H,145,146)(H,147,148)(H,149,150)(H,151,152) |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 2-{[3-({[(5-amino-3,4-dihydroxyoxan-2-yl)oxy](hydroxy)phosphoryl}oxy)-6-{[6-({[(5-amino-3,4-dihydroxyoxan-2-yl)oxy](hydroxy)phosphoryl}oxy)-5-[(1,3-dihydroxytetradecylidene)amino]-3-hydroxy-4-[(3-hydroxytetradecanoyl)oxy]oxan-2-yl]methoxy}-5-{[3-(dodecanoyloxy)-1-hydroxytetradecylidene]amino}-4-{[3-(tetradecanoyloxy)tetradecanoyl]oxy}oxan-2-yl]methoxy}-4-{[2-carboxy-6-(1,2-dihydroxyethyl)-4,5-dihydroxyoxan-2-yl]oxy}-6-(1,2-dihydroxyethyl)-5-hydroxyoxane-2-carboxylic acid |
|---|
|
Traditional IUPAC Name: |
2-[(3-{[(5-amino-3,4-dihydroxyoxan-2-yl)oxy(hydroxy)phosphoryl]oxy}-6-[(6-{[(5-amino-3,4-dihydroxyoxan-2-yl)oxy(hydroxy)phosphoryl]oxy}-5-[(1,3-dihydroxytetradecylidene)amino]-3-hydroxy-4-[(3-hydroxytetradecanoyl)oxy]oxan-2-yl)methoxy]-5-{[3-(dodecanoyloxy)-1-hydroxytetradecylidene]amino}-4-{[3-(tetradecanoyloxy)tetradecanoyl]oxy}oxan-2-yl)methoxy]-4-{[2-carboxy-6-(1,2-dihydroxyethyl)-4,5-dihydroxyoxan-2-yl]oxy}-6-(1,2-dihydroxyethyl)-5-hydroxyoxane-2-carboxylic acid |
|---|
| SMILES: | CCCCCCCCCCCCCC(=O)OC(CCCCCCCCCCC)CC(=O)OC1C(OP(O)(=O)OC2OCC(N)C(O)C2O)C(COC2(CC(OC3(CC(O)C(O)C(O3)C(O)CO)C(O)=O)C(O)C(O2)C(O)CO)C(O)=O)OC(OCC2OC(OP(O)(=O)OC3OCC(N)C(O)C3O)C(N=C(O)CC(O)CCCCCCCCCCC)C(OC(=O)CC(O)CCCCCCCCCCC)C2O)C1N=C(O)CC(CCCCCCCCCCC)OC(=O)CCCCCCCCCCC |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as acylaminosugars. These are organic compounds containing a sugar linked to a chain through N-acyl group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organooxygen compounds |
|---|
|
Class |
Carbohydrates and carbohydrate conjugates |
|---|
| Sub Class | Aminosaccharides |
|---|
|
Direct Parent |
Acylaminosugars |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Oligosaccharide phosphate
- Oligosaccharide
- Hexacarboxylic acid or derivatives
- Acylaminosugar
- Saccharolipid
- Hexose phosphate
- N-acyl-alpha-hexosamine
- C-glucuronide
- C-glycosyl compound
- Glycosyl compound
- O-glycosyl compound
- Pyran carboxylic acid or derivatives
- Pyran carboxylic acid
- Beta-hydroxy acid
- Fatty acid ester
- Ketal
- Dialkyl phosphate
- Fatty amide
- Fatty acyl
- Hydroxy acid
- N-acyl-amine
- Pyran
- Alkyl phosphate
- Organic phosphate
- Organic phosphoric acid derivative
- Oxane
- Phosphoric acid ester
- Secondary alcohol
- Carboxamide group
- 1,2-diol
- Carboxylic acid ester
- Secondary carboxylic acid amide
- 1,2-aminoalcohol
- Amino acid
- Amino acid or derivatives
- Oxacycle
- Carboxylic acid amide
- Organoheterocyclic compound
- Carboxylic acid derivative
- Carboxylic acid
- Acetal
- Primary amine
- Organic oxide
- Carbonyl group
- Hydrocarbon derivative
- Amine
- Alcohol
- Primary aliphatic amine
- Organonitrogen compound
- Primary alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Membrane |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|