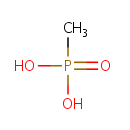
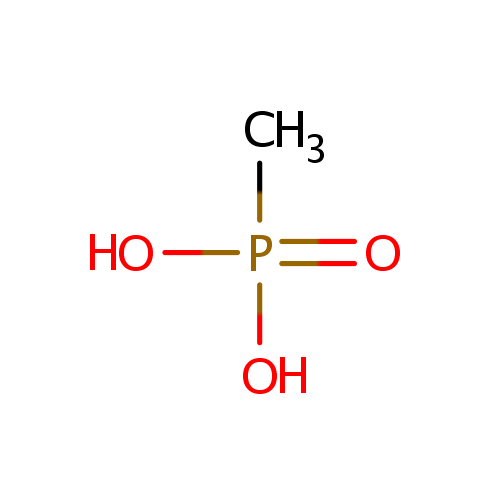
Methylphosphonate (PAMDB001160)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB001160 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Methylphosphonate | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Methylphosphonate is a member of the chemical class known as Organic Phosphonic Acids and Derivatives. These are organic compounds containing phosphonic acid or a derivative thereof. . Phosphonates (Pn) are a large class of organophosphorus molecules that have direct carbon-phosphorus (C-P) bonds in place of the carbon-oxygen-phosphorus ester bond. In bacteria two pathways exist for Pn breakdown for use as a P source: the phosphonatase and C-P lyase pathways. These pathways differ both in regard to their substrate specificity and their cleavage mechanism. The phosphonatase pathway acts on the natural Pn alpha-aminoethylphosphonate (AEPn). In a two-step process it leads to cleavage of the C-P bond by a hydrolysis reaction requiring an adjacent carbonyl group. In contrast the C-P lyase pathway has a broad substrate specificity. It leads to cleavage of substituted Pn (such as AEPn) as well as unsubstituted Pn by a mechanism involving redox or radical chemistry. Due to its broad substrate specificity, the C-P lyase pathway is generally thought to be responsible for the breakdown of Pn herbicides (such as glyphosate) by bacteria. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | CH5O3P | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 96.0224 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 95.997630538 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | YACKEPLHDIMKIO-UHFFFAOYSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/CH5O3P/c1-5(2,3)4/h1H3,(H2,2,3,4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 993-13-5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | methylphosphonic acid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | methylphosphonic acid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CP(O)(O)=O | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as organic phosphonic acids. These are organic compounds containing phosphonic acid. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organophosphorus compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Organic phosphonic acids and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Organic phosphonic acids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Organic phosphonic acids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 108.5 °C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||