|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000946 |
|---|
|
Identification |
|---|
| Name: |
5-Amino-4-imidazolecarboxyamide |
|---|
| Description: | 5-Amino-4-imidazolecarboxyamide is a metabolite of purine metabolism. Adenine phosphoribosyltransferase [EC:2.4.2.7] catalyzes both the formation of 5-Amino-4-imidazolecarboxyamide and its conversion to 1-(5'-Phosphoribosyl)-5-amino-4-imidazolecarboxamide, also called AICAR. (KEGG) |
|---|
|
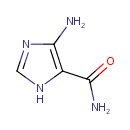
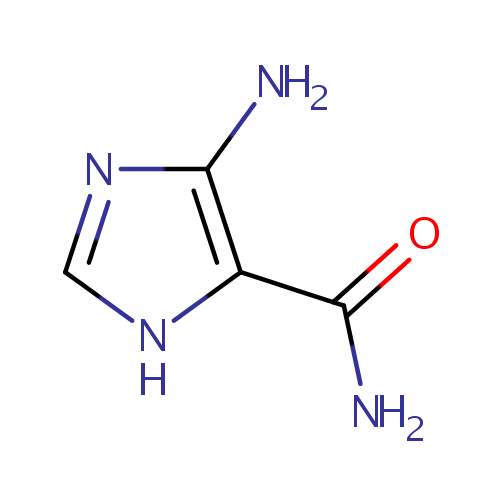
Structure |
|
|---|
| Synonyms: | - 1H-Imidazole-4-carboxamide, 5-amino-, monohydrochloride
- 360-97-4 (FREE BASE)
- 4-Amino-1H-imidazole-5-carboxamide
- 4-Amino-5-carboxamido imidazole hydrochloride
- 4-Amino-5-imidazole carboxamide
- 4-Amino-5-Imidazolecarboxamide
- 4-Amino-5-imidazolecarboxamide hydrochloride
- 4-Aminoimidazole-5-carboxamide
- 4-Aminoimidazole-5-carboxamide hydrochoride
- 4-Carbamoyl-5-aminoimidazole
- 4-Carboxamido-5-aminoimidazole
- 5'-P-Ribosyl-5-amino-4-imidazole carboxamide
- 5'-Phosphoribosyl-5-amino-4-imidazole carboxamide
- 5(OR 4)-AMINO-IMIDAZOLE-4(OR 5)-CARBOXAMIDE
- 5-Amino-1-(5-phospho-D-ribosyl)imidazole-4-carboxamide
- 5-Amino-1H-Imidazole-4-carboxamide
- 5-Amino-4-imidazolecarboxamide
- 5-Amino-4-imidazolecarboxamide ribotide
- 5-Amino-Imidazole-4-carboxamide
- 5-Aminoimidazol-4-carboxamide
- 5-Aminoimidazol-4-carboxamide, hydrochloride
- 5-Aminoimidazole carboxamide
- 5-Aminoimidazole-4-carboxamide
- 5-Aminoimidazole-4-carboxamide hydrochloride
- 5-Aminoimidazole-4-carboxamide ribotide
- 5-Aminoimidazolecarboxamide
- 5-Imidazolecarboxamide, 4-amino-, hydrochloride
- AIC
- Aic .cntdot. HCL
- AICA
- AICA ribonucleotide
- Aicar
- Aminoimidazole carboxamide
- Colahepat
- Diazol-c
- Imidazole C-4,5 deriv. 2
- Imidazole-4-carboxamide, 5-amino-, monohydrochloride
- WLN: T5M CNJ DVZ EZ
- Z-Nucleotide
|
|---|
|
Chemical Formula: |
C4H6N4O |
|---|
| Average Molecular Weight: |
126.1166 |
|---|
| Monoisotopic Molecular
Weight: |
126.054160834 |
|---|
| InChI Key: |
DVNYTAVYBRSTGK-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C4H6N4O/c5-3-2(4(6)9)7-1-8-3/h1H,5H2,(H2,6,9)(H,7,8) |
|---|
| CAS
number: |
72-40-2 |
|---|
| IUPAC Name: | 4-amino-1H-imidazole-5-carboxamide |
|---|
|
Traditional IUPAC Name: |
4-aminoimidazole-5-carboxamide |
|---|
| SMILES: | NC(=O)C1=C(N)N=CN1 |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as aminoimidazoles. These are organic compounds containing an amino group linked to an imidazole ring. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Azoles |
|---|
| Sub Class | Imidazoles |
|---|
|
Direct Parent |
Aminoimidazoles |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Imidolactam
- Primary aromatic amine
- Aminoimidazole
- Heteroaromatic compound
- Vinylogous amide
- Primary carboxylic acid amide
- Carboxamide group
- Azacycle
- Carboxylic acid derivative
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Amine
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|