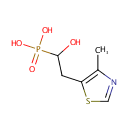
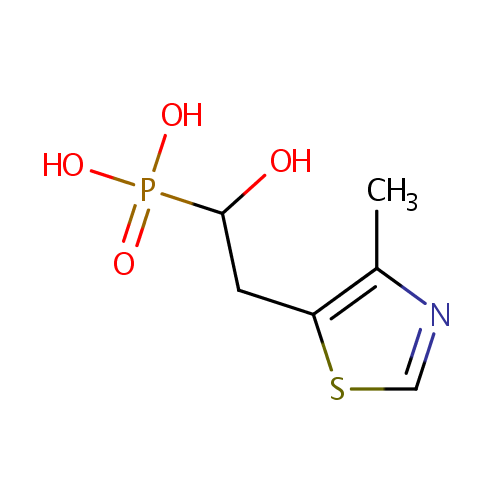
4-Methyl-5-(2-phosphoethyl)-thiazole (PAMDB000942)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000942 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 4-Methyl-5-(2-phosphoethyl)-thiazole | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | 4-methyl-5-(2-phosphoethyl)-thiazole is a member of the chemical class known as Organophosphate Esters. These are organic compounds containing phosphoric acid ester functional group. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C6H10NO4PS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 223.187 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 223.006815015 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | BAAGZOYOQNMHKY-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C6H10NO4PS/c1-4-5(13-3-7-4)2-6(8)12(9,10)11/h3,6,8H,2H2,1H3,(H2,9,10,11) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | [1-hydroxy-2-(4-methyl-1,3-thiazol-5-yl)ethyl]phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | 1-hydroxy-2-(4-methyl-1,3-thiazol-5-yl)ethylphosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC1=C(CC(O)P(O)(O)=O)SC=N1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as 4,5-disubstituted thiazoles. These are compounds containing a thiazole ring substituted at positions 4 and 5 only. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Azoles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Thiazoles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | 4,5-disubstituted thiazoles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Adenosine triphosphate + Dehydroglycine + 1-Deoxy-D-xylulose 5-phosphate + Hydrogen ion + IscS with bound sulfur + NADPH > 4-Methyl-5-(2-phosphoethyl)-thiazole + Adenosine monophosphate + Carbon dioxide +2 Water + IscS sulfur acceptor protein + NADP + Pyrophosphate 5-(2-Hydroxyethyl)-4-methylthiazole + Adenosine triphosphate + 4-methyl-5-(2-hydroxyethyl)thiazole <> 4-Methyl-5-(2-phosphoethyl)-thiazole + ADP + Hydrogen ion 2-Methyl-4-amino-5-hydroxymethylpyrimidine diphosphate + 4-Methyl-5-(2-phosphoethyl)-thiazole + Hydrogen ion <> Pyrophosphate + Thiamine monophosphate 2-Methyl-4-amino-5-hydroxymethylpyrimidine diphosphate + 4-Methyl-5-(2-phosphoethyl)-thiazole <> Pyrophosphate + Thiamine monophosphate Adenosine triphosphate + 5-(2-Hydroxyethyl)-4-methylthiazole <> ADP + 4-Methyl-5-(2-phosphoethyl)-thiazole C15815 + L-Tyrosine + Iminoglycine <> 4-Methyl-5-(2-phosphoethyl)-thiazole Adenosine triphosphate + 5-(2-Hydroxyethyl)-4-methylthiazole > Hydrogen ion + ADP + 4-Methyl-5-(2-phosphoethyl)-thiazole | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||