|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000919 |
|---|
|
Identification |
|---|
| Name: |
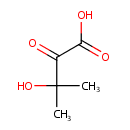
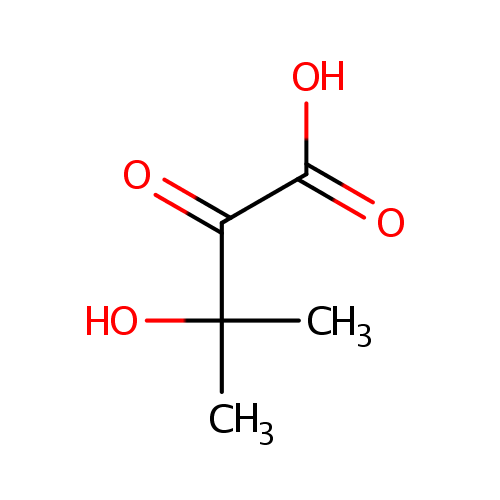
3-Hydroxy-3-methyl-2-oxobutanoic acid |
|---|
| Description: | 3-hydroxy-3-methyl-2-oxobutanoic acid belongs to the class of Branched Fatty Acids. These are fatty acids containing a branched chain. (inferred from compound structure)α-Acetolactic acid (α-acetolactate) is a precursor in the biosynthesis of the branched chain amino acids valine and leucine. α-Acetolactic acid is produced from two molecules of pyruvic acid by acetolactate synthase. α-Acetolactic acid can also be decarboxylated by alpha-acetolactate decarboxylase to produce acetoin. (WikiPedia) |
|---|
|
Structure |
|
|---|
| Synonyms: | - 2-Oxo-3-hydroxyisovalerate
- 2-Oxo-3-hydroxyisovaleric acid
- 3-Hydroxy-3-methyl-2-oxo-butanoate
- 3-Hydroxy-3-methyl-2-oxo-butanoic acid
- 3-Hydroxy-3-methyl-2-oxobutanoate
|
|---|
|
Chemical Formula: |
C5H8O4 |
|---|
| Average Molecular Weight: |
132.1146 |
|---|
| Monoisotopic Molecular
Weight: |
132.042258744 |
|---|
| InChI Key: |
DNOPJXBPONYBLB-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C5H8O4/c1-5(2,9)3(6)4(7)8/h9H,1-2H3,(H,7,8) |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 3-hydroxy-3-methyl-2-oxobutanoic acid |
|---|
|
Traditional IUPAC Name: |
2-oxo-3-hydroxyisovaleric acid |
|---|
| SMILES: | CC(C)(O)C(=O)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as hydroxy fatty acids. These are fatty acids in which the chain bears a hydroxyl group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
|
Direct Parent |
Hydroxy fatty acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Hydroxy fatty acid
- Methyl-branched fatty acid
- Short-chain keto acid
- Branched fatty acid
- Beta-hydroxy acid
- Keto acid
- Hydroxy acid
- Alpha-keto acid
- Acyloin
- Tertiary alcohol
- Alpha-hydroxy ketone
- Ketone
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Valine, leucine and isoleucine biosynthesis pae00290
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Yurtsever D. (2007). Fatty acid methyl ester profiling of Enterococcus and Esherichia coli for microbial source tracking. M.sc. Thesis. Villanova University: U.S.A
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | 17667 | | HMDB ID | Not Available | | Pubchem Compound ID | 440248 | | Kegg ID | C04181 | | ChemSpider ID | 389229 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|