|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000903 |
|---|
|
Identification |
|---|
| Name: |
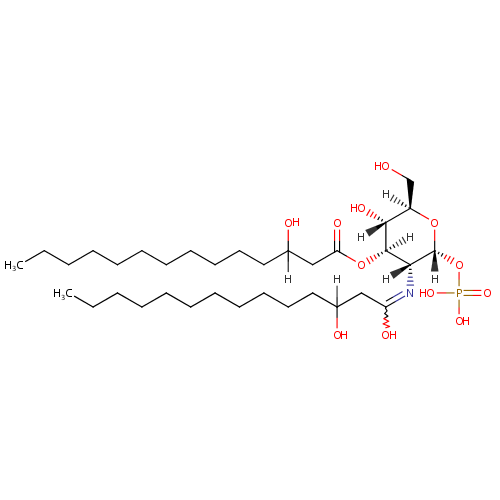
2,3-Bis(3-hydroxytetradecanoyl)-beta-D-glucosaminyl 1-phosphate |
|---|
| Description: | 2,3-bis(3-hydroxytetradecanoyl)-beta-D-glucosaminyl 1-phosphate is a member of the chemical class known as Hexoses. These are monosaccharides in which the sugar unit is a hexose. Lipid X is a diacylglucosamine 1-phosphate bearing beta-hydroxymyristoyl groups at positions 2 and 3. (PMID 2998869) The simplest of these, lipid X, is a derivative of glucosamine-1-phosphate substituted with beta-hydroxymyristoyl moieties at positions 2 and 3. (PMID 6382553) Lipid X (2,3-diacylglucosamine-1-phosphate) is a novel monosaccharide precursor of lipid A that has some of the physiologic activities of endotoxin but little toxicity. Perhaps because lipid X is a subunit of lipid A, lipid X shows a partial pyrogenic effect while also decreasing the pyrogenic activity of complete lipopolysaccharide (LPS). Lipid X is a potential prototype compound for a new type of chemotherapy directed at blocking the harmful effects of LPS during bacterial septicemia. (PMID 3308707) A monosaccharide precursor of Pseudomonas aeruginosa lipid A, designated lipid X, which is a diacylglucosamine 1-phosphate with beta-hydroxymyristoyl groups at positions 2 and 3, was shown to have the ability to induce the production of tumor necrosis factor (TNF)-like tumor-cytotoxic factor by a murine macrophage-like cell line, J774. (PMID 3701065) |
|---|
|
Structure |
|
|---|
| Synonyms: | - 2,3-bis(β-hydroxymyristoyl)-α-D-glucosaminyl 1-phosphate
- 2,3-Bis(β-hydroxymyristoyl)-α-D-glucosaminyl 1-phosphoric acid
- 2,3-bis(3-hydroxymyristoyl)-α-D-glucosaminyl 1-phosphate
- 2,3-Bis(3-hydroxymyristoyl)-α-D-glucosaminyl 1-phosphoric acid
- 2,3-Bis(3-hydroxymyristoyl)-a-D-glucosaminyl 1-phosphate
- 2,3-Bis(3-hydroxymyristoyl)-a-D-glucosaminyl 1-phosphoric acid
- 2,3-Bis(3-hydroxymyristoyl)-alpha-D-glucosaminyl 1-phosphate
- 2,3-Bis(3-hydroxymyristoyl)-alpha-D-glucosaminyl 1-phosphoric acid
- 2,3-Bis(3-hydroxymyristoyl)-α-D-glucosaminyl 1-phosphate
- 2,3-Bis(3-hydroxymyristoyl)-α-D-glucosaminyl 1-phosphoric acid
- 2,3-bis(3-hydroxytetradecanoyl)-α-D-glucosaminyl 1-phosphate
- 2,3-Bis(3-hydroxytetradecanoyl)-α-D-glucosaminyl 1-phosphoric acid
- 2,3-Bis(3-hydroxytetradecanoyl)-a-D-glucosaminyl 1-phosphate
- 2,3-Bis(3-hydroxytetradecanoyl)-a-D-glucosaminyl 1-phosphoric acid
- 2,3-Bis(3-hydroxytetradecanoyl)-alpha-D-glucosaminyl 1-phosphate
- 2,3-Bis(3-hydroxytetradecanoyl)-alpha-D-glucosaminyl 1-phosphoric acid
- 2,3-Bis(3-hydroxytetradecanoyl)-b-D-glucosaminyl 1-phosphate
- 2,3-Bis(3-hydroxytetradecanoyl)-b-D-glucosaminyl 1-phosphoric acid
- 2,3-Bis(3-hydroxytetradecanoyl)-beta-D-glucosaminyl 1-phosphoric acid
- 2,3-Bis(3-hydroxytetradecanoyl)-α-D-glucosaminyl 1-phosphate
- 2,3-Bis(3-hydroxytetradecanoyl)-α-D-glucosaminyl 1-phosphoric acid
- 2,3-Bis(3-hydroxytetradecanoyl)-β-D-glucosaminyl 1-phosphate
- 2,3-Bis(3-hydroxytetradecanoyl)-β-D-glucosaminyl 1-phosphoric acid
- 2,3-Bis(b-hydroxymyristoyl)-a-D-glucosaminyl 1-phosphate
- 2,3-Bis(b-hydroxymyristoyl)-a-D-glucosaminyl 1-phosphoric acid
- 2,3-Bis(beta-hydroxymyristoyl)-alpha-D-glucosaminyl 1-phosphate
- 2,3-Bis(beta-hydroxymyristoyl)-alpha-D-glucosaminyl 1-phosphoric acid
- 2,3-Bis(β-hydroxymyristoyl)-α-D-glucosaminyl 1-phosphate
- 2,3-Bis(β-hydroxymyristoyl)-α-D-glucosaminyl 1-phosphoric acid
- BHMyrGc1P
- Lipid x
- Monosaccharide precursor, e coli lipid a
|
|---|
|
Chemical Formula: |
C34H66NO12P |
|---|
| Average Molecular Weight: |
711.8611 |
|---|
| Monoisotopic Molecular
Weight: |
711.432263093 |
|---|
| InChI Key: |
HEHQDWUWJVPREQ-JIMHHRIFSA-N |
|---|
| InChI: | InChI=1S/C34H66NO12P/c1-3-5-7-9-11-13-15-17-19-21-26(37)23-29(39)35-31-33(32(41)28(25-36)45-34(31)47-48(42,43)44)46-30(40)24-27(38)22-20-18-16-14-12-10-8-6-4-2/h26-28,31-34,36-38,41H,3-25H2,1-2H3,(H,35,39)(H2,42,43,44)/t26?,27?,28-,31-,32-,33-,34-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 3-hydroxy-N-[(2R,3R,4R,5S,6R)-5-hydroxy-6-(hydroxymethyl)-4-[(3-hydroxytetradecanoyl)oxy]-2-(phosphonooxy)oxan-3-yl]tetradecanimidic acid |
|---|
|
Traditional IUPAC Name: |
3-hydroxy-N-[(2R,3R,4R,5S,6R)-5-hydroxy-6-(hydroxymethyl)-4-[(3-hydroxytetradecanoyl)oxy]-2-(phosphonooxy)oxan-3-yl]tetradecanimidic acid |
|---|
| SMILES: | [H]C(O)(CCCCCCCCCCC)CC(=O)O[C@@]1([H])[C@]([H])(O)[C@@]([H])(CO)O[C@]([H])(OP(O)(O)=O)[C@]1([H])N=C(O)CC([H])(O)CCCCCCCCCCC |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as saccharolipids. These are compounds in which fatty acids are linked directly to a sugar backbone, forming structures that are compatible with membrane bilayers. In the saccharolipids, a sugar substitutes for the glycerol backbone that is present in glycerolipids and glycerophospholipids. The most familiar saccharolipids contain an acylated glucosamine. In contrast to others glycolipids, the fatty acid is not glycosidically linked to the sugar moiety. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Saccharolipids |
|---|
| Sub Class | Not Available |
|---|
|
Direct Parent |
Saccharolipids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Saccharolipid
- N-acyl-alpha-hexosamine
- Glucosamine
- Amino sugar
- Monosaccharide phosphate
- Monoalkyl phosphate
- Amino saccharide
- Fatty acid ester
- Beta-hydroxy acid
- Fatty acyl
- Alkyl phosphate
- Phosphoric acid ester
- Oxane
- Organic phosphoric acid derivative
- Organic phosphate
- Monosaccharide
- Hydroxy acid
- Saccharide
- Secondary alcohol
- Carboxylic acid ester
- Oxacycle
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Carboximidic acid derivative
- Carboximidic acid
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -3 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Membrane |
|---|
| Reactions: | 2,3-Bis(3-hydroxytetradecanoyl)-beta-D-glucosaminyl 1-phosphate + UDP-2,3-Bis(3-hydroxytetradecanoyl)glucosamine <> Hydrogen ion + 2,3,2',3'-Tetrakis(3-hydroxytetradecanoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1-phosphate + Uridine 5'-diphosphateWater + UDP-2,3-Bis(3-hydroxytetradecanoyl)glucosamine <>2 Hydrogen ion + 2,3-Bis(3-hydroxytetradecanoyl)-beta-D-glucosaminyl 1-phosphate + Uridine 5'-monophosphateUDP-2,3-Bis(3-hydroxytetradecanoyl)glucosamine + Water <> Uridine 5'-monophosphate + 2,3-Bis(3-hydroxytetradecanoyl)-beta-D-glucosaminyl 1-phosphateUDP-2,3-Bis(3-hydroxytetradecanoyl)glucosamine + 2,3-Bis(3-hydroxytetradecanoyl)-beta-D-glucosaminyl 1-phosphate <> Uridine 5'-diphosphate + 2,3,2',3'-Tetrakis(3-hydroxytetradecanoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1-phosphate |
|---|
|
Pathways: |
- Lipopolysaccharide biosynthesis pae00540
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Amano, F., Nishijima, M., Akagawa, K., Akamatsu, Y. (1985). "Enhancement of O2- generation and tumoricidal activity of murine macrophages by a monosaccharide precursor of Escherichia coli lipid A." FEBS Lett 192:263-266. Pubmed: 2998869
- Amano, F., Nishijima, M., Akamatsu, Y. (1986). "A monosaccharide precursor of Escherichia coli lipid A has the ability to induce tumor-cytotoxic factor production by a murine macrophage-like cell line, J774.1." J Immunol 136:4122-4127. Pubmed: 3701065
- Golenbock, D. T., Will, J. A., Raetz, C. R., Proctor, R. A. (1987). "Lipid X ameliorates pulmonary hypertension and protects sheep from death due to endotoxin." Infect Immun 55:2471-2476. Pubmed: 3308707
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Raetz, C. R. (1984). "The enzymatic synthesis of lipid A: molecular structure and biologic function of monosaccharide precursors." Rev Infect Dis 6:463-471. Pubmed: 6382553
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|