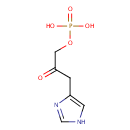
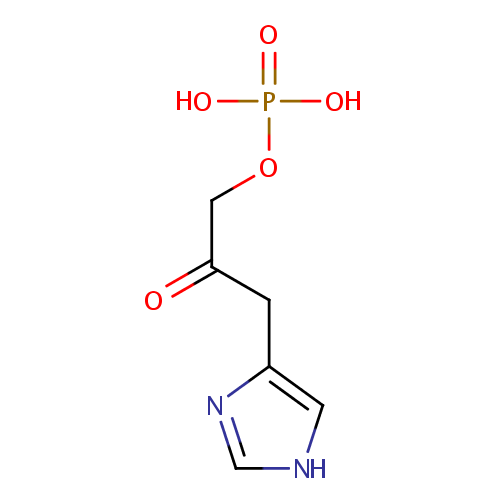
| References: |
- Albritton WL, Levin AP: Some comparative kinetic data on the enzyme imidazoleacetol phosphate:L-glutamate aminotransferase derived from mutant strains of Salmonella typhimurium. J Biol Chem. 1970 May 25;245(10):2525-8. Pubmed: 5445798
- AMES BN, HORECKER BL: The biosynthesis of histidine: imidazoleacetol phosphate transaminase. J Biol Chem. 1956 May;220(1):113-28. Pubmed: 13319331
- AMES BN, MITCHELL HK: The biosynthesis of histidine; imidazoleglycerol phosphate, imidazoleacetol phosphate, and histidinol phosphate. J Biol Chem. 1955 Feb;212(2):687-96. Pubmed: 14353870
- Henderson GB, Snell EE: Vitamin B 6 -responsive histidine deficiency in mutants of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2903-7. Pubmed: 4943547
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- LEVIN AP, HARTMAN PE: ACTION OF A HISTIDINE ANALOGUE, 1,2,4-TRIAZOLE-3-ALANINE, IN SALMONELLA TYPHIMURIUM. J Bacteriol. 1963 Oct;86:820-8. Pubmed: 14066480
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
|
|---|