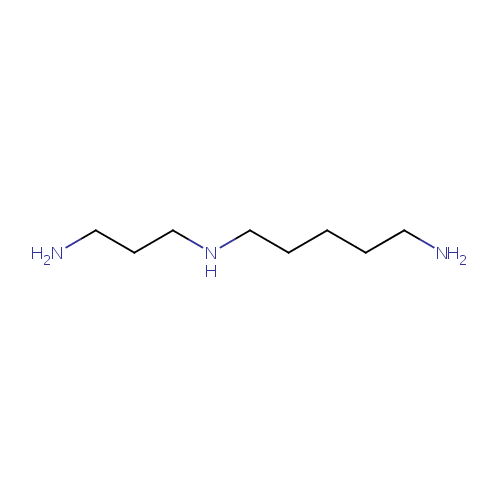
| Description: | Aminopropylcadaverine,a polyamine, is the final product of aminopropylcadaverine biosynthesis pathway. Polyamines are important for cell growth and are believed to be involved in many processes including DNA, RNA, and protein synthesis, as well as membrane integrity and resistance to stress, to name a few. Cadaverine and aminopropylcadaverine are alternative polyamines that can at least partially substitute for purtrescine and spermidine, the primary polyamines found in Pseudomonas aeruginosa. Lysine is decarboxylated to form cadaverine which is then converted to aminopropylcadaverine by the aminopropyltransferase, SpeE. |
|---|
| References: |
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
|
|---|