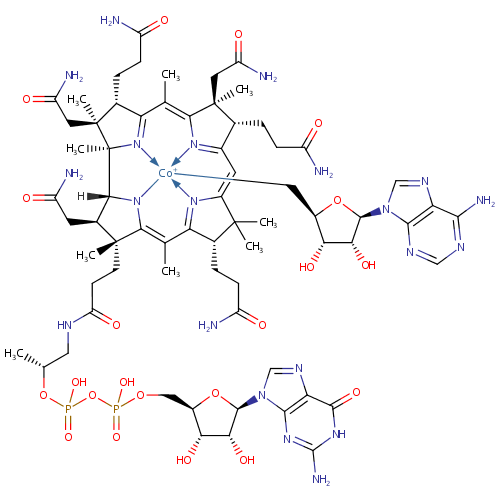
Adenosylcobinamide-GDP (PAMDB000826)
| Record Information | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | |||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000826 | |||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||
| Name: | Adenosylcobinamide-GDP | |||||||||||||||||||||||||||||||||||||||
| Description: | Adenosylcobinamide-GDP,a known de novo intermediate, is involved in Porphyrin and chlorophyll metabolism.In Salmonella typhimurium LT2, under anaerobic conditions, CobU (EC 2.7.7.62 and EC 2.7.1.156), CobT (EC 2.4.2.21), CobC (EC 3.1.3.73) and CobS (EC 2.7.8.26) catalyse reactions in the nucleotide loop | |||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| |||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C68H97CoN21O21P2 | |||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 1665.5066 | |||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 1664.597512489 | |||||||||||||||||||||||||||||||||||||||
| InChI Key: | IQTYKHRKNGVJEO-FGHWVWCISA-M | |||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C58H86N16O18P2.C10H12N5O3.Co/c1-25(91-94(87,88)92-93(85,86)89-23-33-45(82)46(83)52(90-33)74-24-67-44-50(74)71-53(65)72-51(44)84)22-66-41(81)16-17-55(6)31(18-38(62)78)49-58(9)57(8,21-40(64)80)30(12-15-37(61)77)43(73-58)27(3)48-56(7,20-39(63)79)28(10-13-35(59)75)32(68-48)19-34-54(4,5)29(11-14-36(60)76)42(69-34)26(2)47(55)70-49;1-4-6(16)7(17)10(18-4)15-3-14-5-8(11)12-2-13-9(5)15;/h19,24-25,28-31,33,45-46,49,52,82-83H,10-18,20-23H2,1-9H3,(H19,59,60,61,62,63,64,65,66,68,69,70,71,72,73,75,76,77,78,79,80,81,84,85,86,87,88);2-4,6-7,10,16-17H,1H2,(H2,11,12,13);/q;;+2/p-1/t25?,28-,29-,30-,31+,33-,45-,46-,49?,52-,55-,56+,57+,58+;4-,6-,7-,10-;/m11./s1 | |||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | |||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | [(1R,2R,3S,4S,8S,9S,14S,18R,19R)-18-(2-{[(2R)-2-({[({[(2R,3S,4R,5R)-5-(2-amino-6-oxo-6,9-dihydro-1H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)propyl]carbamoyl}ethyl)-4,9,14-tris(2-carbamoylethyl)-3,8,19-tris(carbamoylmethyl)-2,3,6,8,13,13,16,18-octamethyl-20,21,22,23-tetraazapentacyclo[15.2.1.1?,??1?????1??,???tricosa-5(23),6,10(22),11,15(21),16-hexaen-20-yl]({[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl})cobaltylium | |||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | [(1R,2R,3S,4S,8S,9S,14S,18R,19R)-18-(2-{[(2R)-2-[({[(2R,3S,4R,5R)-5-(2-amino-6-oxo-1H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy(hydroxy)phosphoryl}oxy(hydroxy)phosphoryl)oxy]propyl]carbamoyl}ethyl)-4,9,14-tris(2-carbamoylethyl)-3,8,19-tris(carbamoylmethyl)-2,3,6,8,13,13,16,18-octamethyl-20,21,22,23-tetraazapentacyclo[15.2.1.1?,??1?????1??,???tricosa-5(23),6,10(22),11,15(21),16-hexaen-20-yl]({[(2S,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl})cobaltylium | |||||||||||||||||||||||||||||||||||||||
| SMILES: | CC(CNC(=O)CC[C@]1(C)[C@@H](CC(N)=O)C2N=C1\C(C)=C1/N=C(/C=C3\N=C(\C(\C)=C4\[C@@H](CCC(N)=O)[C@](C)(CC(N)=O)[C@@]2(C)N4[Co+]C[C@H]2O[C@H]([C@H](O)[C@@H]2O)N2C=NC4=C2N=CN=C4N)[C@@](C)(CC(N)=O)[C@@H]3CCC(N)=O)C(C)(C)[C@@H]1CCC(N)=O)OP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)N1C=NC2=C1N=C(N)NC2=O | |||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as purine ribonucleoside diphosphates. These are purine ribobucleotides with diphosphate group linked to the ribose moiety. | |||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||
| Super Class | Nucleosides, nucleotides, and analogues | |||||||||||||||||||||||||||||||||||||||
| Class | Purine nucleotides | |||||||||||||||||||||||||||||||||||||||
| Sub Class | Purine ribonucleotides | |||||||||||||||||||||||||||||||||||||||
| Direct Parent | Purine ribonucleoside diphosphates | |||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| |||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | |||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||
| State: | Solid | |||||||||||||||||||||||||||||||||||||||
| Charge: | 2 | |||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | |||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| |||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | |||||||||||||||||||||||||||||||||||||||
| Reactions: | Adenosylcobinamide-GDP + N1-(alpha-D-ribosyl)-5,6-dimethyl-benzimidazole > Adenosylcobalamin + Guanosine monophosphate + Hydrogen ion Adenosyl cobinamide phosphate + Guanosine triphosphate + Hydrogen ion > Adenosylcobinamide-GDP + Pyrophosphate Adenosyl cobinamide phosphate + Guanosine triphosphate <> Adenosylcobinamide-GDP + Pyrophosphate Adenosylcobalamin + Guanosine monophosphate <> Adenosylcobinamide-GDP + N1-(alpha-D-ribosyl)-5,6-dimethyl-benzimidazole Adenosylcobinamide-GDP + N1-(5-Phospho-a-D-ribosyl)-5,6-dimethylbenzimidazole > adenosylcobalamin 5'-phosphate + Guanosine monophosphate + Hydrogen ion Adenosylcobinamide-GDP + N1-(alpha-D-ribosyl)-5,6-dimethyl-benzimidazole + N1-(5-Phospho-a-D-ribosyl)-5,6-dimethylbenzimidazole <> Guanosine monophosphate + Adenosylcobalamin + Adenosylcobalamin 5'-phosphate adenosylcobinamide phosphate + Guanosine triphosphate + Hydrogen ion > Pyrophosphate + Adenosylcobinamide-GDP + Adenosylcobinamide-GDP N1-(5-Phospho-a-D-ribosyl)-5,6-dimethylbenzimidazole + Adenosylcobinamide-GDP + Adenosylcobinamide-GDP > Hydrogen ion + GMP + Adenosylcobalamin 5'-phosphate | |||||||||||||||||||||||||||||||||||||||
| Pathways: | ||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||
| Spectra: | ||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||
| References: |
| |||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | |||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | |||||||||||||||||||||||||||||||||||||||
| Links | ||||||||||||||||||||||||||||||||||||||||
| External Links: |
| |||||||||||||||||||||||||||||||||||||||