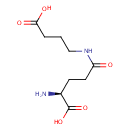
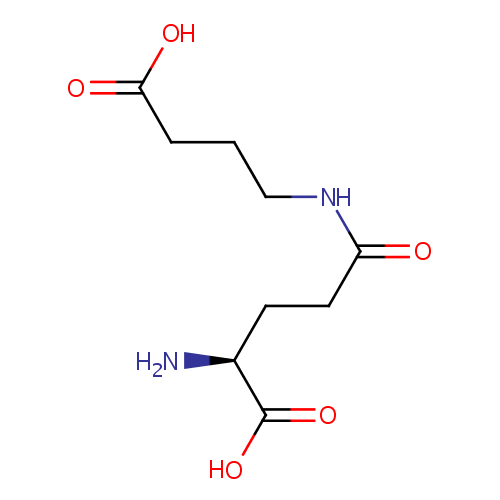
4-(Glutamylamino) butanoate (PAMDB000824)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000824 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 4-(Glutamylamino) butanoate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | 4-(Glutamylamino) butanoate is a polyamine that is an intermediate in putrescine degradation . Polyamines (the most common of which are putrescine , spermidine , and spermine ), a group of positively charged small molecules present in virtually all living organisms, have been implicated in many biological processes, including binding to nucleic acids, stabilizing membranes, and stimulating several enzymes. Although polyamines are clearly necessary for optimal cell growth, a surplus of polyamines can cause inhibition of growth and protein synthesis, and thus a balance is desired between the production and breakdown of polyamines. In putrescine degradation , 4-(Glutamylamino) butanoate is a substrate for gamma-glutamyl-gamma-aminobutyrate hydrolase (puuD) and can be generated from the hydrolysis of gamma-glutamyl-gamma-aminobutyraldehyde. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C9H16N2O5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 232.2337 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 232.105921632 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | MKYPKZSGLSOGLL-LURJTMIESA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C9H16N2O5/c10-6(9(15)16)3-4-7(12)11-5-1-2-8(13)14/h6H,1-5,10H2,(H,11,12)(H,13,14)(H,15,16)/t6-/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 5105-96-4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (2S)-2-amino-4-[(3-carboxypropyl)carbamoyl]butanoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | glugaba | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | N[C@@H](CCC(=O)NCCCC(O)=O)C(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as gamma amino acids and derivatives. These are amino acids having a (-NH2) group attached to the gamma carbon atom. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | ################ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | ################ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Gamma amino acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 4-(Glutamylamino) butanoate + Water <> gamma-Aminobutyric acid + L-Glutamate gamma-Glutamyl-gamma-butyraldehyde + Water + NADP <> 4-(Glutamylamino) butanoate +2 Hydrogen ion + NADPH gamma-Glutamyl-gamma-butyraldehyde + NAD + Water <> 4-(Glutamylamino) butanoate + NADH + Hydrogen ion NAD(P)<sup>+</sup> + gamma-Glutamyl-gamma-butyraldehyde + Water > 4-(Glutamylamino) butanoate + NAD(P)H + Hydrogen ion L-Glutamic acid + Hydrogen ion + L-Glutamate > Carbon dioxide + 4-(Glutamylamino) butanoate 4-(γ-glutamylamino)butanal + Water + NADP > 4-(Glutamylamino) butanoate +2 Hydrogen ion + NADPH + NADPH 4-(Glutamylamino) butanoate + Water > L-Glutamic acid + gamma-Aminobutyric acid + L-Glutamate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||