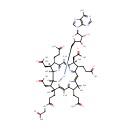
Adenosyl cobinamide (PAMDB000706)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000706 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Adenosyl cobinamide | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Adenosyl cobinamide is an intermediate in Porphyrin and chlorophyll metabolism. It is the third to last step in the synthesis of vitamin B12 coenzyme and is converted from adenosyl cobyrinate hexaamide via the enzyme cobalamin biosynthetic protein CobC. It is then converted to adenosyl cobinamide phosphate via the enzyme adenosylcobinamide kinase / adenosylcobinamide-phosphate guanylyltransferase [EC:2.7.1.156 and EC 2.7.7.62]. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C58H84CoN16O11 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 1240.3214 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 1239.583747804 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | PAUPKJHCFVPREX-VUCSARQQSA-M | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C48H73N11O8.C10H12N5O3.Co/c1-23(60)22-55-38(67)16-17-45(6)29(18-35(52)64)43-48(9)47(8,21-37(54)66)28(12-15-34(51)63)40(59-48)25(3)42-46(7,20-36(53)65)26(10-13-32(49)61)30(56-42)19-31-44(4,5)27(11-14-33(50)62)39(57-31)24(2)41(45)58-43;1-4-6(16)7(17)10(18-4)15-3-14-5-8(11)12-2-13-9(5)15;/h19,23,26-29,43,60H,10-18,20-22H2,1-9H3,(H14,49,50,51,52,53,54,55,56,57,58,59,61,62,63,64,65,66,67);2-4,6-7,10,16-17H,1H2,(H2,11,12,13);/q;;+3/p-1/t23-,26-,27-,28-,29+,43-,45-,46+,47+,48+;;/m1../s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | {[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl}[(1R,2R,3S,4S,8S,9S,14S,18R,19R)-4,9,14-tris(2-carbamoylethyl)-3,8,19-tris(carbamoylmethyl)-18-(2-{[(2R)-2-hydroxypropyl]carbamoyl}ethyl)-2,3,6,8,13,13,16,18-octamethyl-20,21,22,23-tetraazapentacyclo[15.2.1.1?,??1?????1??,???tricosa-5(23),6,10(22),11,15(21),16-hexaen-20-yl]cobaltylium | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | {[(2S,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl}[(1R,2R,3S,4S,8S,9S,14S,18R,19R)-4,9,14-tris(2-carbamoylethyl)-3,8,19-tris(carbamoylmethyl)-18-(2-{[(2R)-2-hydroxypropyl]carbamoyl}ethyl)-2,3,6,8,13,13,16,18-octamethyl-20,21,22,23-tetraazapentacyclo[15.2.1.1?,??1?????1??,???tricosa-5(23),6,10(22),11,15(21),16-hexaen-20-yl]cobaltylium | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | C[C@@H](O)CNC(=O)CC[C@]1(C)[C@@H](CC(=O)N)[C@@]2([H])N([Co++]CC3OC(C(O)C3O)N3C=NC4=C3N=CN=C4N)\C1=C(C)/C1=N/C(=C\C3=N\C(=C(C)/C4=N[C@]2(C)[C@@](C)(CC(=O)N)[C@@H]4CCC(=O)N)\[C@@](C)(CC(=O)N)[C@@H]3CCC(=O)N)/C(C)(C)[C@@H]1CCC(=O)N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as metallotetrapyrroles. These are polycyclic compounds containing a tetrapyrrole skeleton combined with a metal atom. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Tetrapyrroles and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Metallotetrapyrroles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Metallotetrapyrroles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Adenosine triphosphate + Cobinamide + Hydrogen ion <> Adenosyl cobinamide + Triphosphate Adenosyl cobinamide + Adenosine triphosphate > Adenosyl cobinamide phosphate + ADP + Hydrogen ion Adenosyl cobinamide + Adenosine triphosphate <> Adenosyl cobinamide phosphate + ADP Adenosyl cobinamide + Guanosine triphosphate <> Adenosyl cobinamide phosphate + Guanosine diphosphate Adenosine triphosphate + Cobinamide <> Triphosphate + Adenosyl cobinamide Cob(I)yrinate a,c diamide + Adenosine triphosphate > Adenosyl cobinamide + Triphosphate Adenosine triphosphate + Cob(I)yrinate a,c diamide + Cobinamide <> Triphosphate + Adenosyl cobyrinate a,c diamide + Adenosyl cobinamide Adenosine triphosphate + Guanosine triphosphate + Adenosyl cobinamide <> Adenosyl cobinamide phosphate + ADP + Guanosine diphosphate Cobinamide + Adenosine triphosphate + Cobinamide > Adenosyl cobinamide + Triphosphate + Adenosyl cobinamide + Triphosphate Adenosyl cobinamide + Adenosine triphosphate + Adenosyl cobinamide > Adenosine diphosphate + Hydrogen ion + adenosylcobinamide phosphate + ADP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||