|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000666 |
|---|
|
Identification |
|---|
| Name: |
Selenocystathionine |
|---|
| Description: | Selenocystathionine is formed from selenohomocysteine by the enzyme cystathionine beta-synthase (EC 4.2.1.22), as a by-product of cystathionine synthesis. It is an intermediate in selenoamine acid metabolism. |
|---|
|
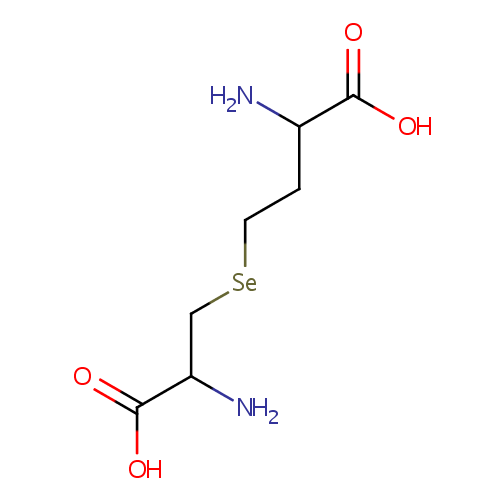
Structure |
|
|---|
| Synonyms: | - 2-Amino-4-((2-amino-2-carboxyethyl)seleno)-Butanoate
- 2-Amino-4-((2-amino-2-carboxyethyl)seleno)-Butanoic acid
- 2-Amino-4-((2-amino-2-carboxyethyl)seleno)butanoate
- 2-Amino-4-((2-amino-2-carboxyethyl)seleno)butanoic acid
- Selenate
- Selenic acid
- Selenocystathionine
|
|---|
|
Chemical Formula: |
C7H14N2O4Se |
|---|
| Average Molecular Weight: |
269.16 |
|---|
| Monoisotopic Molecular
Weight: |
270.011878774 |
|---|
| InChI Key: |
ZNWYDQPOUQRDLY-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C7H14N2O4Se/c8-4(6(10)11)1-2-14-3-5(9)7(12)13/h4-5H,1-3,8-9H2,(H,10,11)(H,12,13) |
|---|
| CAS
number: |
2196-58-9 |
|---|
| IUPAC Name: | 2-amino-4-[(2-amino-2-carboxyethyl)selanyl]butanoic acid |
|---|
|
Traditional IUPAC Name: |
selenocystathionine |
|---|
| SMILES: | NC(CC[Se]CC(N)C(O)=O)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as alpha amino acids. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon). |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
|
Direct Parent |
Alpha amino acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha-amino acid
- Amino fatty acid
- Fatty acyl
- Fatty acid
- Dicarboxylic acid or derivatives
- Selenoether
- Carboxylic acid
- Hydrocarbon derivative
- Primary amine
- Organoselenium compound
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
58 °C |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Esaki N, Nakamura T, Tanaka H, Suzuki T, Morino Y, Soda K: Enzymatic synthesis of selenocysteine in rat liver. Biochemistry. 1981 Jul 21;20(15):4492-6. Pubmed: 6456763
- Freeman JL, Zhang LH, Marcus MA, Fakra S, McGrath SP, Pilon-Smits EA: Spatial imaging, speciation, and quantification of selenium in the hyperaccumulator plants Astragalus bisulcatus and Stanleya pinnata. Plant Physiol. 2006 Sep;142(1):124-34. Epub 2006 Aug 18. Pubmed: 16920881
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Neuhierl, B., Thanbichler, M., Lottspeich, F., Bock, A. (1999). "A family of S-methylmethionine-dependent thiol/selenol methyltransferases. Role in selenium tolerance and evolutionary relation." J Biol Chem 274:5407-5414. Pubmed: 10026151
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|