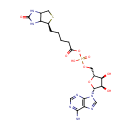
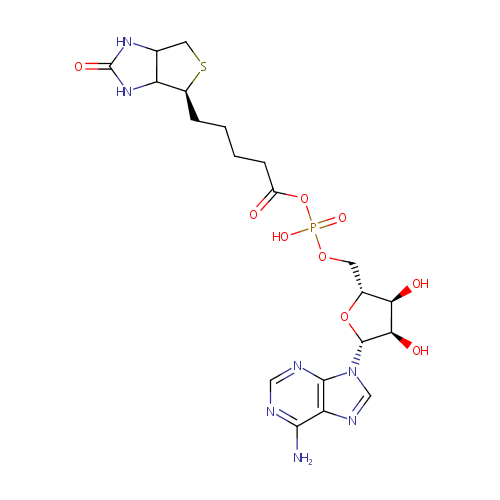
Biotinyl-5'-AMP (PAMDB000655)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000655 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Biotinyl-5'-AMP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | 5'-biotinyl-AMP (B-AMP) is the active form of biotin. Biotin is essential to maintain metabolic homeostasis and as regulator of gene expression. The vitamin biotin plays an essential role in gluconeogenesis, fatty acid synthesis, and carbohydrate metabolism because of its role as cofactor of five carboxylases; pyruvate carboxylase (PC), propionyl-CoA carboxylase (PCC), methylcrotonyl-CoA carboxylase, and two forms of acetyl-CoA carboxylase (ACC-1 and ACC-2). Carboxylase biotinylation is catalyzed by the enzyme holocarboxylase synthetase (HCS) through a reaction that involves the transformation of biotin into B-AMP and its subsequent attachment to a specific lysine residue in the carboxylases. B-AMP is also required to activate a signal transduction cascade that includes a soluble guanylate cyclase (sGC) and cGMP-dependent protein kinase (PKG). The regulatory role of biotin in the biotin cycle seems to be limited to the expression of proteins involved in the transport and utilization of exogenous vitamin while having no effect on biotinidase mRNA levels, enzyme responsible for biotin recycling during carboxylase turnover. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C20H28N7O9PS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 573.517 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 573.140682731 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | UTQCSTJVMLODHM-CFYKWCKVSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C20H28N7O9PS/c21-17-14-18(23-7-22-17)27(8-24-14)19-16(30)15(29)10(35-19)5-34-37(32,33)36-12(28)4-2-1-3-11-13-9(6-38-11)25-20(31)26-13/h7-11,13,15-16,19,29-30H,1-6H2,(H,32,33)(H2,21,22,23)(H2,25,26,31)/t9?,10-,11+,13?,15-,16-,19-/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 4130-20-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}({5-[(4S)-2-oxo-hexahydro-1H-thieno[3,4-d]imidazolidin-4-yl]pentanoyl}oxy)phosphinic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | [(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy({5-[(4S)-2-oxo-hexahydrothieno[3,4-d]imidazolidin-4-yl]pentanoyl}oxy)phosphinic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](COP(O)(=O)OC(=O)CCCC[C@@H]2SCC3NC(=O)NC23)[C@@H](O)[C@H]1O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as purine ribonucleoside monophosphates. These are nucleotides consisting of a purine base linked to a ribose to which one monophosphate group is attached. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Nucleosides, nucleotides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Purine nucleotides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Purine ribonucleotides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Purine ribonucleoside monophosphates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Adenosine triphosphate + Biotin <> Pyrophosphate + Biotinyl-5'-AMP Biotinyl-5'-AMP + Apo-[carboxylase] <> Adenosine monophosphate + Holo-[carboxylase] Adenosine triphosphate + Biotin > Biotinyl-5'-AMP + Pyrophosphate Biotinyl-5'-AMP > Biotin-Carboxyl Carrying Protein Biotin + Adenosine triphosphate > diphosphate + Biotinyl-5'-AMP + Pyrophosphate Biotinyl-5'-AMP + apo-[carboxylase] > Adenosine monophosphate + Biotin-Carboxyl Carrying Protein | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Christner, James E.; Coon, Minor J. Synthesis of (+)-biotinyl 5'-adenylate. Methods Enzymol. (1970), 18(Pt. A), 386-90. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||