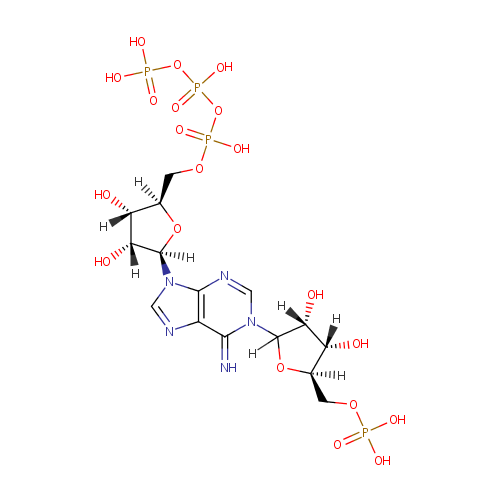
Phosphoribosyl-ATP (PAMDB000631)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000631 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Phosphoribosyl-ATP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Phosphoribosyl-ATP takes part in the Histidine Metabolism pathway.[KEGG ID C02739]. Specifically, Phosphoribosyl-ATP is substrate for phosphoribosyl pyrophosphate synthetase 1. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C15H26N5O20P4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 720.2835 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 720.012159345 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | KYTPWZMUSLPBJZ-UHFFFAOYSA-O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C15H25N5O20P4/c16-12-7-13(18-4-19(12)14-10(23)8(21)5(37-14)1-35-41(25,26)27)20(3-17-7)15-11(24)9(22)6(38-15)2-36-43(31,32)40-44(33,34)39-42(28,29)30/h3-6,8-11,14-16,21-24H,1-2H2,(H6,25,26,27,28,29,30,31,32,33,34)/p+1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | ({[({[(2R,3S,4R,5R)-5-{1-[(3R,4S,5R)-3,4-dihydroxy-5-[(phosphonooxy)methyl]oxolan-2-yl]-6-imino-6,9-dihydro-1H-purin-9-yl}-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | Phosphoribosyl-ATP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | NC1=[N+](C=NC2=C1N=CN2C1OC(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)C(O)C1O)C1OC(COP(O)(O)=O)C(O)C1O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as purine ribonucleoside triphosphates. These are purine ribobucleotides with a triphosphate group linked to the ribose moiety. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Nucleosides, nucleotides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Purine nucleotides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Purine ribonucleotides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Purine ribonucleoside triphosphates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Adenosine triphosphate + Phosphoribosyl pyrophosphate <> Pyrophosphate + Phosphoribosyl-ATP Water + Phosphoribosyl-ATP <> Hydrogen ion + Pyrophosphate + Phosphoribosyl-AMP Phosphoribosyl-ATP + Water <> Phosphoribosyl-AMP + Pyrophosphate Phosphoribosyl pyrophosphate + Hydrogen ion > Pyrophosphate + Phosphoribosyl-ATP + Phosphoribosyl-ATP Phosphoribosyl-ATP + Water + Phosphoribosyl-ATP > Hydrogen ion + Pyrophosphate + Phosphoribosyl-AMP (2R,4S)-2-methyl-2,3,3,4-tetrahydroxytetrahydrofuran + Phosphoribosyl-ATP + Water + (2R,4S)-2-Methyl-2,3,3,4-tetrahydroxytetrahydrofuran + Phosphoribosyl-ATP > (2R,4S)-2-methyl-2,3,3,4-tetrahydroxytetrahydrofuran + Adenosine diphosphate + Hydrogen ion + Pyrophosphate + ADP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in phosphoribosyl-AMP cyclohydrolase activity
- Specific function:
- 1-(5-phosphoribosyl)-ATP + H(2)O = 1-(5- phosphoribosyl)-AMP + diphosphate
- Gene Name:
- hisI
- Locus Tag:
- PA5066
- Molecular weight:
- 15.4 kDa
Reactions
| 1-(5-phosphoribosyl)-ATP + H(2)O = 1-(5-phosphoribosyl)-AMP + diphosphate. |
| 1-(5-phosphoribosyl)-AMP + H(2)O = 1-(5-phosphoribosyl)-5-((5-phosphoribosylamino)methylideneamino)imidazole-4-carboxamide. |
- General function:
- Involved in ATP phosphoribosyltransferase activity
- Specific function:
- Catalyzes the condensation of ATP and 5-phosphoribose 1- diphosphate to form N'-(5'-phosphoribosyl)-ATP (PR-ATP). Has a crucial role in the pathway because the rate of histidine biosynthesis seems to be controlled primarily by regulation of hisG enzymatic activity
- Gene Name:
- hisG
- Locus Tag:
- PA4449
- Molecular weight:
- 22.8 kDa
Reactions
| 1-(5-phospho-D-ribosyl)-ATP + diphosphate = ATP + 5-phospho-alpha-D-ribose 1-diphosphate. |