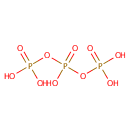
Triphosphate (PAMDB000496)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000496 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Triphosphate | ||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | A triphosphate is a salt or ester containing three phosphate groups. It is the ionic form of triphosphoric acid, a condensed form of phosphoric acid. Triphosphate is an intermediate in the biosynthesis of Folate, the metabolism of purine, the metabolism of Porphyrin, the metabolism of Pyrimidine and the metabolism of Thiamine. The cleavage of the high energy triphosphate bonds in ATP (to ADP or AMP) is the central route of generating energy in cells. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | O10P3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 252.9153 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 252.870430756 | ||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | UNXRWKVEANCORM-UHFFFAOYSA-I | ||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/H5O10P3/c1-11(2,3)9-13(7,8)10-12(4,5)6/h(H,7,8)(H2,1,2,3)(H2,4,5,6)/p-5 | ||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 14127-68-5 | ||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | {[hydroxy(phosphonooxy)phosphoryl]oxy}phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | tripolyphosphate | ||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | [O-]P([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O | ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of inorganic compounds known as non-metal pyrophosphates. These are inorganic non-metallic compoundscontaining a pyrophosphate as its largest oxoanion. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Inorganic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Homogeneous non-metal compounds | ||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Non-metal oxoanionic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Non-metal pyrophosphates | ||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Non-metal pyrophosphates | ||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Guanosine triphosphate + Water > Guanosine + Triphosphate dGTP + Water <> Deoxyguanosine + Triphosphate Adenosine triphosphate + Cob(I)alamin + Hydrogen ion <> Adenosylcobalamin + Triphosphate Adenosine triphosphate + Cobinamide + Hydrogen ion <> Adenosyl cobinamide + Triphosphate Adenosine triphosphate + Pyrophosphate <> ADP + Triphosphate Dihydroneopterin triphosphate + Water > Acetaldehyde + 6-Carboxy-5,6,7,8-tetrahydropterin + Hydrogen ion + Triphosphate Adenosine triphosphate + Cob(I)alamin <> Triphosphate + Adenosylcobalamin Dihydroneopterin triphosphate <> Dyspropterin + Triphosphate Cob(I)yrinate a,c diamide + Adenosine triphosphate <> Adenosyl cobyrinate a,c diamide + Triphosphate Adenosine triphosphate + Cobinamide <> Triphosphate + Adenosyl cobinamide Cob(I)yrinate a,c diamide + Adenosine triphosphate > Adenosyl cobinamide + Triphosphate Water + dGTP > Hydrogen ion + Triphosphate + Deoxyguanosine Adenosine triphosphate + Cob(I)yrinate a,c diamide > Triphosphate + Adenosylcob(III)yrinic acid a,c-diamide Dihydroneopterin triphosphate + Water > 6-Carboxy-5,6,7,8-tetrahydropterin + Acetaldehyde + Triphosphate Adenosine triphosphate + Cob(I)yrinate a,c diamide + Cobinamide <> Triphosphate + Adenosyl cobyrinate a,c diamide + Adenosyl cobinamide Triphosphate + Water <> Pyrophosphate + Phosphate Cobinamide + Adenosine triphosphate + Cobinamide > Adenosyl cobinamide + Triphosphate + Adenosyl cobinamide + Triphosphate Cobalamin + Adenosine triphosphate + vitamin B12 > coenzyme B12 + Triphosphate + Polyphosphate More...Adenosine triphosphate + Cobalamin + vitamin B12 > Triphosphate + Adenosylcobalamin + Triphosphate 7,8-dihydroneopterin 3'-triphosphate + Water > Acetaldehyde + Triphosphate +2 Hydrogen ion + 6-Carboxy-5,6,7,8-tetrahydropterin + Triphosphate | ||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Tsuhako, Mitsutomo; Sueyoshi, Chiyoko; Miyajima, Tohru; Ohashi, Shigeru; Nariai, Hiroyuki; Motooka, Itaru. The reaction of cyclo-triphosphate with ethanolamines. Bulletin of the Chemical Society of Japan (1986), 59(10), 3091-5. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||