|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000469 |
|---|
|
Identification |
|---|
| Name: |
5-Methylcytosine |
|---|
| Description: | 5-Methylcytosine is a methylated form of cytosine in which a methyl group is attached to carbon 5, altering its structure without altering its base-pairing properties. 5-Methylcytosine is an epigenetic modification formed by the action of DNA methyltransferases. Its function varies significantly among species. In bacteria, 5-methylcytosine can be found at a variety of sites, and is often used as a marker to protect DNA from being cut by native methylation-sensitive restriction enzymes. (Wikipedia) |
|---|
|
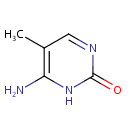
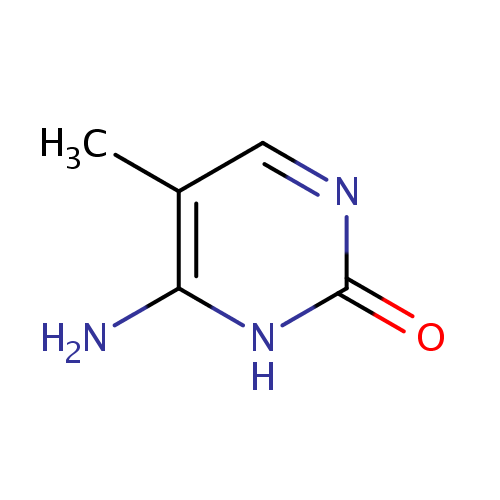
Structure |
|
|---|
| Synonyms: | - 2(1H)-Pyrimidinone, 4-amino-5-methyl-
- 4-Amino-5-methyl-2(1H)-Pyrimidinone
- 4-Amino-5-methyl-2-(1H)-Pyrimidinone
- 4-Amino-5-methyl-2-pyrimidinol
- 5-Methyl-Cytosine
- 5-Methylcytosine
- 5-Methylcytosine>96
- Cytosine, 5-methyl-
- Cytosine, 5-methyl- (VAN)
|
|---|
|
Chemical Formula: |
C5H7N3O |
|---|
| Average Molecular Weight: |
125.1286 |
|---|
| Monoisotopic Molecular
Weight: |
125.058911861 |
|---|
| InChI Key: |
LRSASMSXMSNRBT-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C5H7N3O/c1-3-2-7-5(9)8-4(3)6/h2H,1H3,(H3,6,7,8,9) |
|---|
| CAS
number: |
554-01-8 |
|---|
| IUPAC Name: | 6-amino-5-methyl-1,2-dihydropyrimidin-2-one |
|---|
|
Traditional IUPAC Name: |
5-methylcytosine |
|---|
| SMILES: | CC1=C(N)NC(=O)N=C1 |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as pyrimidones. These are compounds that contain a pyrimidine ring,which bears a ketone. Pyrimidine is a 6-membered ring consisting of four carbon atoms and two nitrogen centers at the 1- and 3- ring positions. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
|
Direct Parent |
Pyrimidones |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pyrimidone
- Aminopyrimidine
- Primary aromatic amine
- Hydropyrimidine
- Heteroaromatic compound
- Azacycle
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Amine
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
270 °C |
|---|
| Experimental Properties: |
| Property | Value | Source |
|---|
| Water Solubility: | 34.5 mg/mL [MERCK INDEX (1996)] | PhysProp |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Pfeifer GP, You YH, Besaratinia A: Mutations induced by ultraviolet light. Mutat Res. 2005 Apr 1;571(1-2):19-31. Epub 2005 Jan 20. Pubmed: 15748635
|
|---|
| Synthesis Reference: |
Umetani, Hideki. Method for preparing 5-methylcytosine. Jpn. Kokai Tokkyo Koho (2006), 8 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|