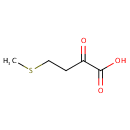
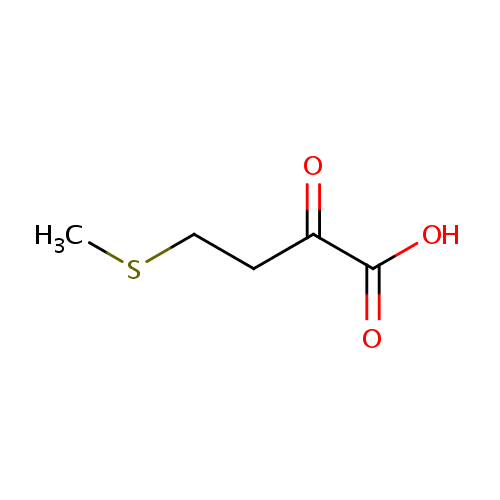
| References: |
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Martensson J: The occurrence of 4-methylthio-2-hydroxybutyrate in human urine. Anal Biochem. 1986 Apr;154(1):43-9. Pubmed: 3706736
- Quash G, Roch AM, Chantepie J, Michal Y, Fournet G, Dumontet C: Methional derived from 4-methylthio-2-oxobutanoate is a cellular mediator of apoptosis in BAF3 lymphoid cells. Biochem J. 1995 Feb 1;305 ( Pt 3):1017-25. Pubmed: 7848263
- Yurtsever D. (2007). Fatty acid methyl ester profiling of Enterococcus and Esherichia coli for microbial source tracking. M.sc. Thesis. Villanova University: U.S.A
|
|---|