|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000387 |
|---|
|
Identification |
|---|
| Name: |
N-Succinyl-L-glutamate |
|---|
| Description: | N-succinyl-L-glutamate is a member of the chemical class known as Tricarboxylic Acids and Derivatives. These are organic compounds containing three carboxylic acid groups (or salt/ester derivatives thereof). |
|---|
|
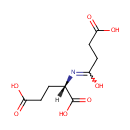
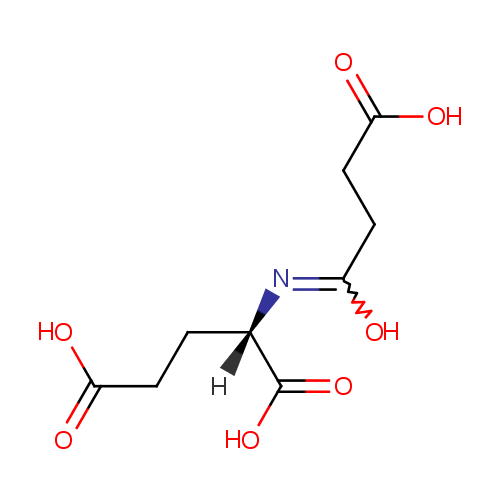
Structure |
|
|---|
| Synonyms: | - (2S)-2-(3-Carboxypropanoylamino)pentanedioate
- (2S)-2-(3-Carboxypropanoylamino)pentanedioic acid
- N-(3-Carboxy-1-oxopropyl)-L-glutamate
- N-(3-Carboxy-1-oxopropyl)-L-glutamic acid
- N-(3-Carboxypropanoyl)-L-glutamate
- N-(3-Carboxypropanoyl)-L-glutamic acid
- N-Succinyl-L-glutamate
- N-Succinyl-L-glutamic acid
- N2-Succinyl-L-glutamate
- N2-Succinyl-L-glutamic acid
- N2SucGlu
|
|---|
|
Chemical Formula: |
C9H13NO7 |
|---|
| Average Molecular Weight: |
247.202 |
|---|
| Monoisotopic Molecular
Weight: |
247.069201775 |
|---|
| InChI Key: |
JCNBNOQGFSXOML-YFKPBYRVSA-N |
|---|
| InChI: | InChI=1S/C9H13NO7/c11-6(2-4-8(14)15)10-5(9(16)17)1-3-7(12)13/h5H,1-4H2,(H,10,11)(H,12,13)(H,14,15)(H,16,17)/t5-/m0/s1 |
|---|
| CAS
number: |
33981-72-5 |
|---|
| IUPAC Name: | (2S)-2-[(3-carboxy-1-hydroxypropylidene)amino]pentanedioic acid |
|---|
|
Traditional IUPAC Name: |
(2S)-2-[(3-carboxy-1-hydroxypropylidene)amino]pentanedioic acid |
|---|
| SMILES: | [H][C@@](CCC(O)=O)(N=C(O)CCC(O)=O)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as n-acyl-aliphatic-alpha amino acids. These are alpha amino acids carrying a N-acylated aliphatic chain. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
|
Direct Parent |
N-acyl-aliphatic-alpha amino acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- N-acyl-aliphatic-alpha amino acid
- Tricarboxylic acid or derivatives
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboxylic acid
- Carboximidic acid derivative
- Carboximidic acid
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -3 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Arginine and proline metabolism pae00330
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | 48957 | | HMDB ID | Not Available | | Pubchem Compound ID | 440847 | | Kegg ID | C05931 | | ChemSpider ID | 389689 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|