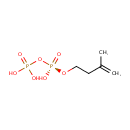
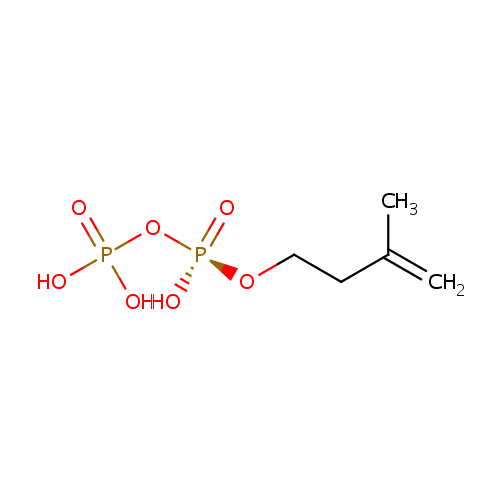
Isopentenyl pyrophosphate (PAMDB000348)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000348 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Isopentenyl pyrophosphate | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Isopentenyl pyrophosphate, IPP or isopentenyl diphosphate, is an intermediate in the HMG-CoA reductase pathway used by organisms in the biosynthesis of terpenes and terpenoids. IPP is formed from Mevalonate-5-pyrophosphate, in a reaction catalyzed by the enzyme mevalonate-5-pyrophosphate decarboxylase. (wikipedia) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C5H12O7P2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 246.0921 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 246.005825762 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | NUHSROFQTUXZQQ-UHFFFAOYSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C5H12O7P2/c1-5(2)3-4-11-14(9,10)12-13(6,7)8/h1,3-4H2,2H3,(H,9,10)(H2,6,7,8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 358-71-4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | ({hydroxy[(3-methylbut-3-en-1-yl)oxy]phosphoryl}oxy)phosphonic acid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | isopentenyl-diphosphate | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC(=C)CCO[P@](O)(=O)OP(O)(O)=O | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as isoprenoid phosphates. These are prenol lipids containing a phosphate group linked to an isoprene (2-methylbuta-1,3-diene) unit. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Lipids and lipid-like molecules | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Prenol lipids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Isoprenoid phosphates | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Isoprenoid phosphates | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Hydrogen ion + 1-Hydroxy-2-methyl-2-butenyl 4-diphosphate + NADH > Water + Isopentenyl pyrophosphate + NAD Farnesyl pyrophosphate + 8 Isopentenyl pyrophosphate >8 Pyrophosphate + Undecaprenyl diphosphate Dimethylallylpyrophosphate + Isopentenyl pyrophosphate > Geranyl-PP + Pyrophosphate Geranyl-PP + Isopentenyl pyrophosphate + Geranyl diphosphate <> Farnesyl pyrophosphate + Pyrophosphate Isopentenyl pyrophosphate <> Dimethylallylpyrophosphate Geranyl-PP + Isopentenyl pyrophosphate <> Pyrophosphate + Farnesyl pyrophosphate 1-Hydroxy-2-methyl-2-butenyl 4-diphosphate + NADPH + Hydrogen ion <> Isopentenyl pyrophosphate + NADP + Water Farnesyl pyrophosphate + 8 Isopentenyl pyrophosphate <> di-trans,poly-cis-Undecaprenyl diphosphate +8 Pyrophosphate + Undecaprenyl diphosphate Farnesyl pyrophosphate + 5 Isopentenyl pyrophosphate <> Octaprenyl diphosphate +5 Pyrophosphate Isopentenyl pyrophosphate + NAD(P)<sup>+</sup> + Water < 1-Hydroxy-2-methyl-2-butenyl 4-diphosphate + NAD(P)H + Hydrogen ion Farnesyl pyrophosphate + Isopentenyl pyrophosphate > all-<i>trans</i>-octaprenyl diphosphate + Pyrophosphate Isopentenyl pyrophosphate + NAD(P)(+) + Water > 1-Hydroxy-2-methyl-2-butenyl 4-diphosphate + NAD(P)H Isopentenyl pyrophosphate + NAD + NADP + Water + Dimethylallylpyrophosphate <> 1-Hydroxy-2-methyl-2-butenyl 4-diphosphate + NADH + NADPH + Hydrogen ion 1-Hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate + Hydrogen ion + NADPH + NADPH > Water + NADPH + Isopentenyl pyrophosphate + Isopentenyl pyrophosphate More...Isopentenyl pyrophosphate + Isopentenyl pyrophosphate <> Dimethylallylpyrophosphate + Dimethylallylpyrophosphate Dimethylallylpyrophosphate + Isopentenyl pyrophosphate + Dimethylallylpyrophosphate + Isopentenyl pyrophosphate > Pyrophosphate + Geranyl-PP + Geranyl-PP Geranyl-PP + Isopentenyl pyrophosphate + Geranyl-PP + Isopentenyl pyrophosphate > Pyrophosphate + Farnesyl pyrophosphate + Farnesyl pyrophosphate Farnesyl pyrophosphate + 8 Isopentenyl pyrophosphate + Farnesyl pyrophosphate + 8 Isopentenyl pyrophosphate >8 Pyrophosphate + di-trans,octa-cis-undecaprenyl diphosphate Farnesyl pyrophosphate + 5 Isopentenyl pyrophosphate + Farnesyl pyrophosphate + 5 Isopentenyl pyrophosphate >5 Pyrophosphate + Octaprenyl diphosphate + Octaprenyl diphosphate | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Kao, Chai-Lin; Kittleman, William; Zhang, Hua; Seto, Haruo; Liu, Hung-Wen. Stereochemical Analysis of Isopentenyl Diphosphate Isomerase Type II from Staphylococcus aureus Using Chemically Synthesized (S)- and (R)-[2-2H]Isopentenyl Diphosphates.Organic Let | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||